
Catalytic upcycling of plastic wastes to valuable chemicals offers the opportunity to simultaneously address the enormous environmental problems associated with plastics and achieve the circular economy1,2. However, the upcycling of plastic wastes containing polyvinyl chloride (PVC) is particularly challenging due to the interference of chlorine, which can be released during PVC depolymerization and deactivate the catalyst3,4.
Here we present a catalytic process for the co-upcycling of PVC and polyethylene terephthalate (PET). By using a chlorine-containing ionic liquid as the catalyst/solvent and ZnCl₂ as Lewis acid catalyst, with in-situ utilization of PVC-released chlorine, we successfully converted PET into terephthalic acid(TPA) and 1,2-dichloroethane(EDC) with high yields. The results reveal that chlorine from PVC, previously considered detrimental to the transformation of other polymers and the cause of catalyst poisoning, can actually have a positive role in the upcycling of plastic wastes.
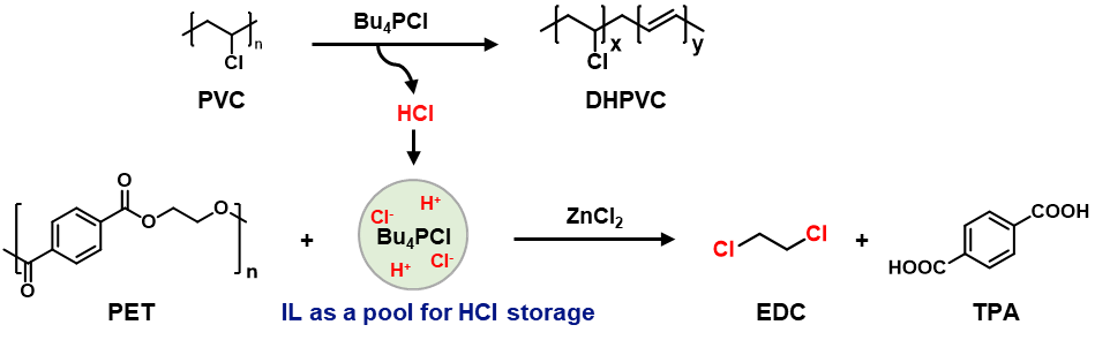
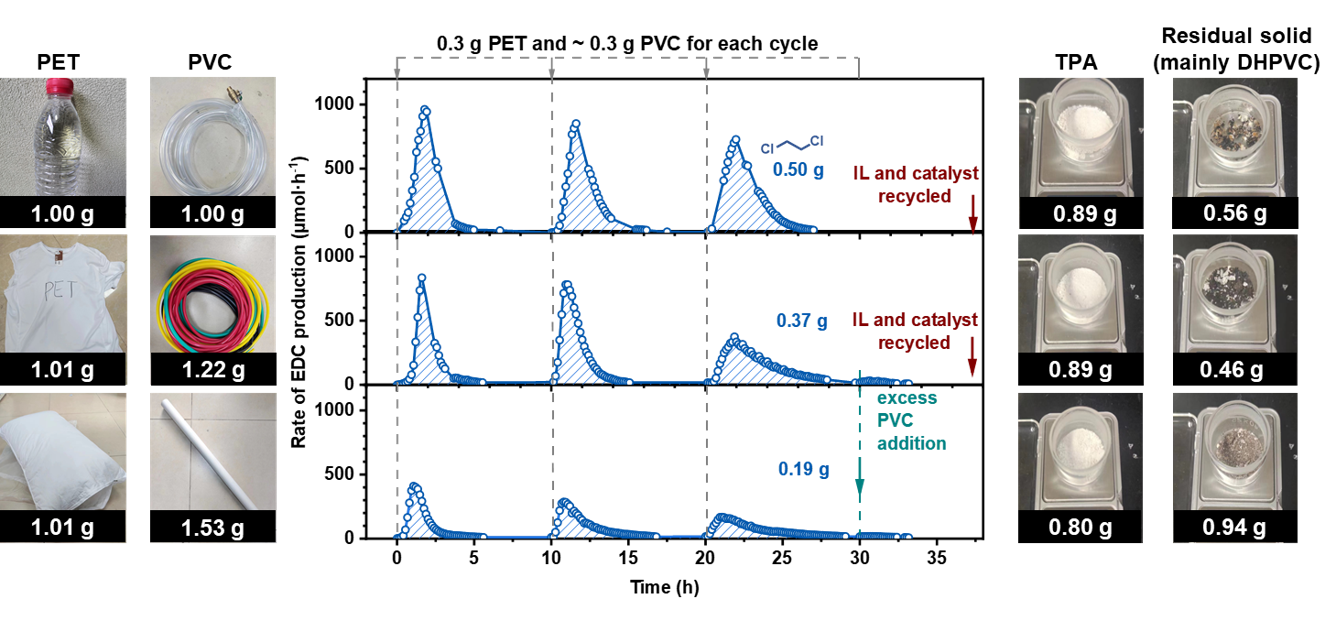
Ionic liquids (ILs) may serve as a suitable medium for this purpose (Figure 2a), as they exhibit low vapor pressures, high thermal stabilities, wide liquid ranges, and reversible gas absorption abilities5,6.It has been reported that IL could be used for PVC dehydrochlorination7,8. Our experiment also verified that PVC can effectively dehydrochlorinate in ionic liquids, and the released HCl can be effectively stored in ionic liquids. The stored HCl can be used for the conversion of PET polyester to produce DCM and TPA effectively. This system is also appliable for real-life plastic products made by PET and PVC. The ionic liquid and catalyst can be recycled for sustained reuse.
For more information, please see our recent publication in Nature Sustainability: https://www.nature.com/articles/s41893-023-01234-1
References
1 Geyer, R. in Plastic Waste and Recycling Vol. Pages 13-32 (ed Trevor M. Letcher) Ch. 2, Pages 13-32 (Academic Press, 2020).
2 Li, H. et al. Expanding plastics recycling technologies: chemical aspects, technology status and challenges. Green Chem. 24, 8899-9002, doi:10.1039/d2gc02588d (2022).
3 Miskolczi, N., Bartha, L. & Angyal, A. Pyrolysis of polyvinyl chloride (PVC)-containing mixed plastic wastes for recovery of hydrocarbons. Energy Fuels 23, 2743-2749, doi:10.1021/ef8011245 (2009).
4 Paci, M. & La Mantia, F. P. Influence of small amounts of polyvinylchloride on the recycling of polyethyleneterephthalate. Polym. Degrad. Stab. 63, 11-14, doi:10.1016/s0141-3910(98)00053-6 (1999).
5 Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun., 2399-2407, doi:10.1039/b107270f (2001).
6 Xiao, Q. et al. Tuning the basicity for highly efficient and reversible hydrogen chloride absorption to develop a green acid scavenger. ACS Sustainable Chem. Eng. 10, 2289-2293, doi:10.1021/acssuschemeng.1c08464 (2022).
7 Lorenzetti, A., Choi, S. Y., Roso, M., Modesti, M. & McNally, T. Effect of dual functional ionic liquids on the thermal degradation of poly(vinyl chloride). Polym. Degrad. Stabil. 129, 12-18, doi:10.1016/j.polymdegradstab.2016.04.001 (2016).
8 Oster, K. et al. Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids. Green Chem. 22, 5132-5142, doi:10.1039/d0gc01312a (2020).
Follow the Topic
-
Nature Sustainability

This journal publishes significant original research from a broad range of natural, social and engineering fields about sustainability, its policy dimensions and possible solutions.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in