Controlling the force in nanomotors
Published in Materials and Cell & Molecular Biology
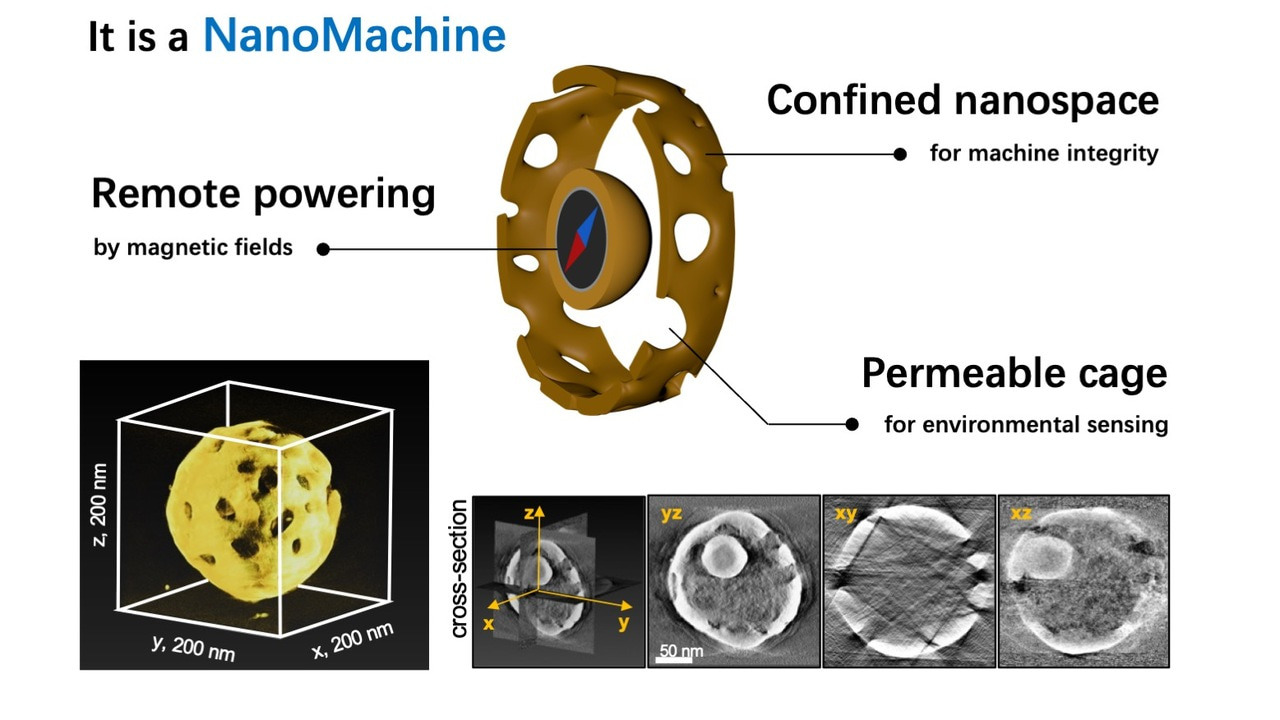
Since Richard Feynman's groundbreaking 1959 lecture, "There is Plenty of Room at the Bottom," the vision of nanomachines serving as non-invasive surgeons to cure diseases within the human body has fascinated scientists. In the wake of Feynman's lecture, nanosciences have flourished, yielding nano-sized systems with autonomous propulsion and controlled navigation capabilities. However, the development of intricate machines with diverse mechanical abilities at the small scale remains a formidable challenge, given the disruptive influence of ,for instance, Brownian motion.
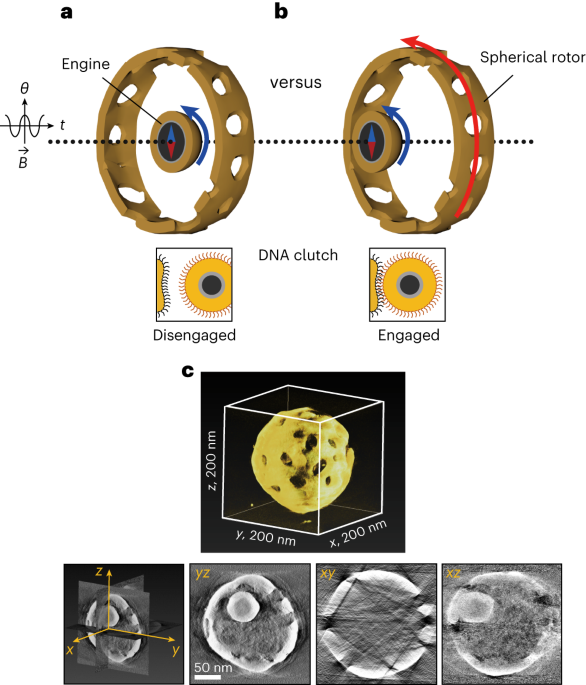
To address these challenges, we devised a strategy involving physical confinement, trapping a magnetic nanoparticle within a porous gold cage. The breakthrough lies in integrating a DNA interface, allowing reversible and environment-triggered force transmission from the magnetic particle to the gold rotor—a nanomachine's clutch system (Figure 1a).
In our study, we crafted a nanomotor utilizing a 45-nm ferromagnetic nanoparticle as the engine and a 200-nm porous gold cage as the spherical rotor. The synthesis is straightforward and provides a high uniformity of the nanomachines (Figure 1b). The engine is powered externally by rotary magnetic fields. DNA interfaces serve as environmental sensors, switching between Brownian motion-driven free engine movement (off state) and locking the engine to the cage (on state) for torque transmission to the rotor.
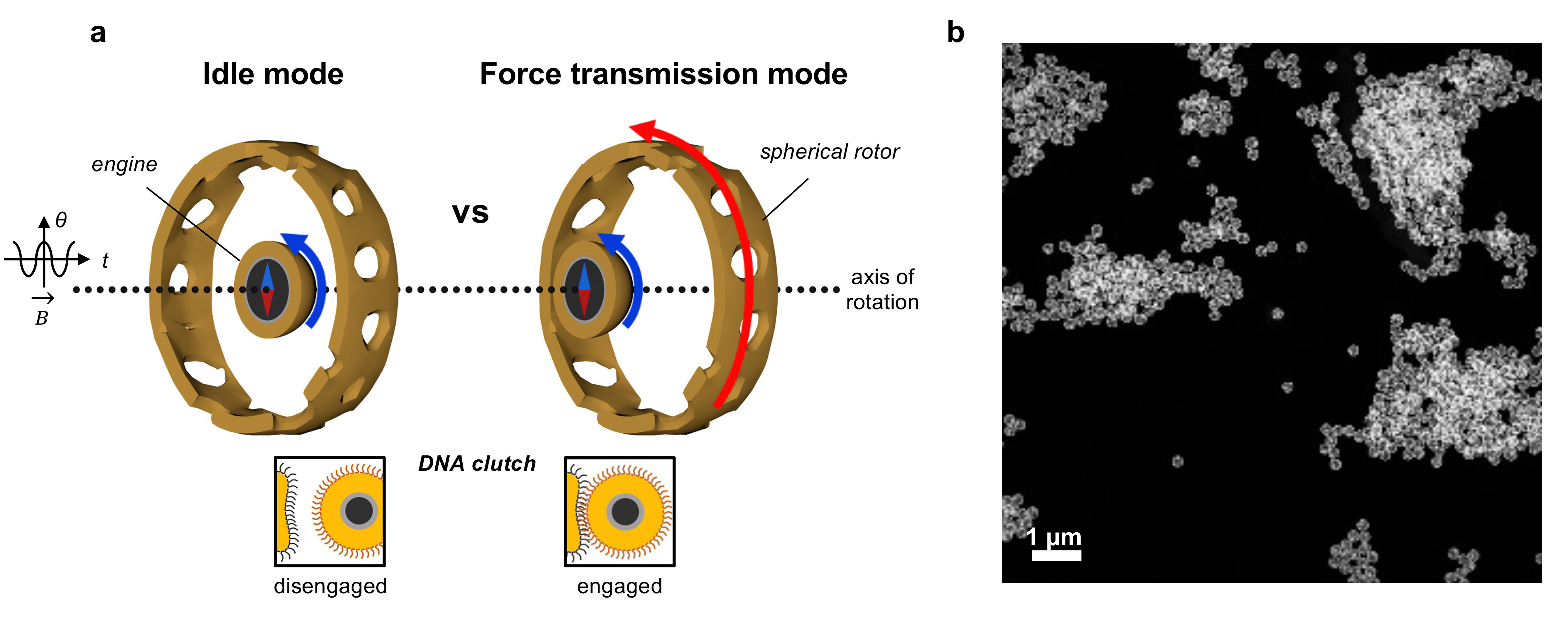
Figure 1. Design of the nanomachine with a clutch. a, Depiction of how the clutch functions. The magnetic engine-particle can reversibly engage with the porous rotor-cage by DNA hybridization. In the idle mode, the clutch is disengaged (‘off’ state), whereas in the force transmission mode (’on’ state), the received torque from the engine is transferred to the rotor. B, magnetic field; θ, rotating angle; t, time. b, Low magnification STEM image of many nanomachines with high uniformity.
In specific environments, such as in the presence of target DNA, a mechanical link forms between the engine and rotor. The cage's porous structure enables real-time responses of the DNA clutch to environmental cues. Even when externally powered, the rotor remains stationary until the correct signal (target DNA) engages the clutch.
To observe these dynamics at the nanoscale, we employed liquid-phase in situ transmission electron microscopy (TEM) with a high-speed camera, achieving a remarkable temporal resolution of 3.48 ms. This meticulous analysis offered crucial insights into the nanomotor's behavior and verified that the engine particle is indeed, randomly moving within the cage when the clutch is disengaged.
Beyond scientific curiosity, our work extends to practical applications. We assessed the nanomachine's performance by anchoring it to a supported lipid bilayer platform and tracking fluorescent beads as optical markers (Figure 2a). This setup allowed us to study controlled rotational work output by observing the circular trajectories of the beads at different frequencies and directions (Figure 2b).
In vitro experiments demonstrated the nanomachine's efficacy in biological settings, where it delivered precise mechanical forces to activate cell receptors like Notch and integrin Figure 2c,d). This activation triggered engineered cellular responses, detectable through fluorescence microscopy (Figure 2e).
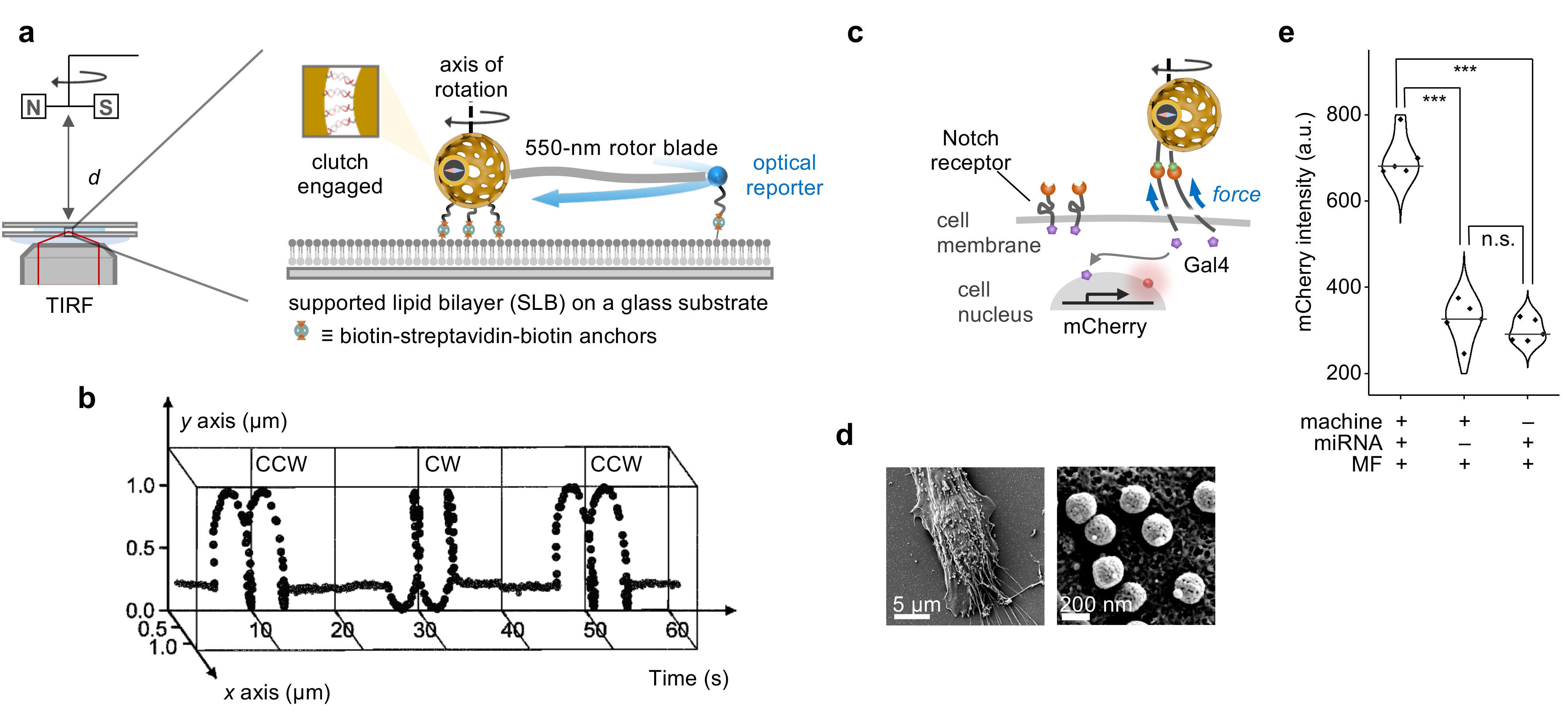
Figure 2. Rotational work output of the nanomachine. a, Experimental set-up for observing the rotational dynamics on a supported lipid bilayer under an inverted TIRF microscope equipped with distance tunable rotary magnetic fields for remote magnetic control. b, The trajectory of the optical reporter attached to the far tip of the rotor blade on the nanomachine under rotary magnetic fields. c, Schematic illustration of the nanomotor’s mechanical activation in vitro of Notch receptors in response to environmental miRNA. d, Scanning electron microscopy images of the labelled nanomotors on the cell. e, Statistical analysis of the mCherry fluorescence intensity of the experimental and control groups.
The incorporation of a clutch mechanism marks a significant advancement compared to previous nanomotors, which lacked the ability to regulate force transmission. With this adaptable and responsive design, our team envisions transformative applications in nanomedicine and beyond.
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in