Convective dissolution of capillary-trapped carbon dioxide
Published in Earth & Environment, Research Data, and Sustainability

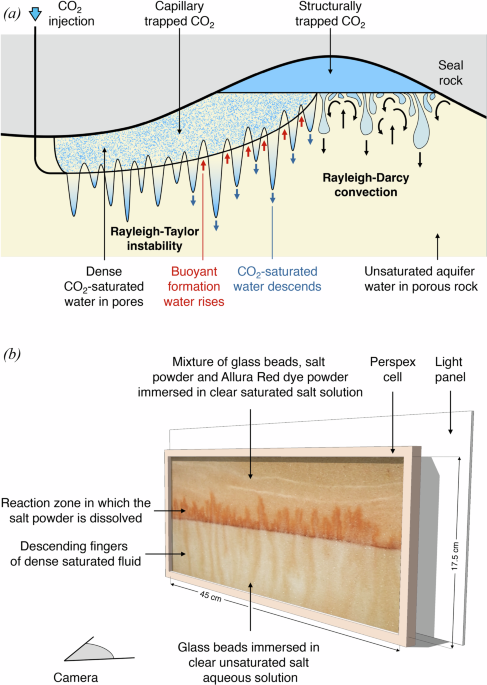
Carbon storage in deep saline aquifers has become an important part of plans to decarbonise the energy system. Typically, aquifers are composed of a porous, permeable rock containing formation water. As carbon dioxide (CO2) is injected into an aquifer, some of it migrates towards topographic highs within the aquifer, while some is retained in the pore spaces of the rock by capillary forces. This leads to zones of porous rock where individual pore spaces contain capillary-trapped CO2.
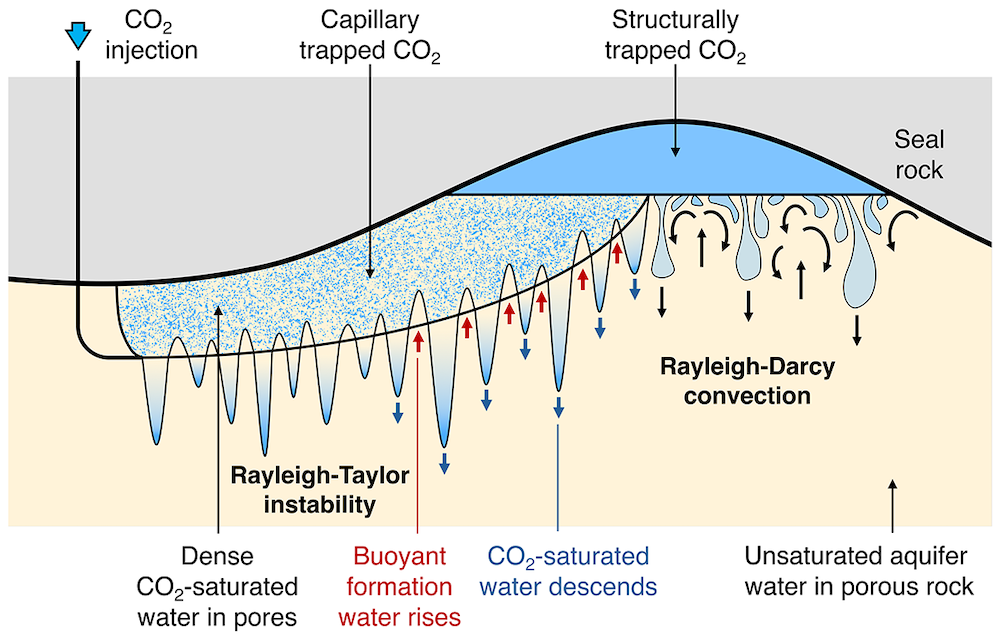
At the Institute for Energy and Environmental Flows in Cambridge, we have developed a new laboratory experiment which models the dissolution of the capillary-trapped CO2. This is an analogue experiment, in which a mixture of glass beads and saline water is used to form a layered porous medium in a thin Hele-Shaw cell, analogous to the porous rock in the aquifer. In the laboratory, some salt powder is added to the bead pack: during an experiment, the salt powder is progressively dissolved by the water in the cell, just like the CO2 is dissolved by aquifer water in the field. Using this analogue experiment enables us to observe the convective flow which develops in the laboratory system and then model the flow which develops in the aquifer, at great depth and over much longer time scales.
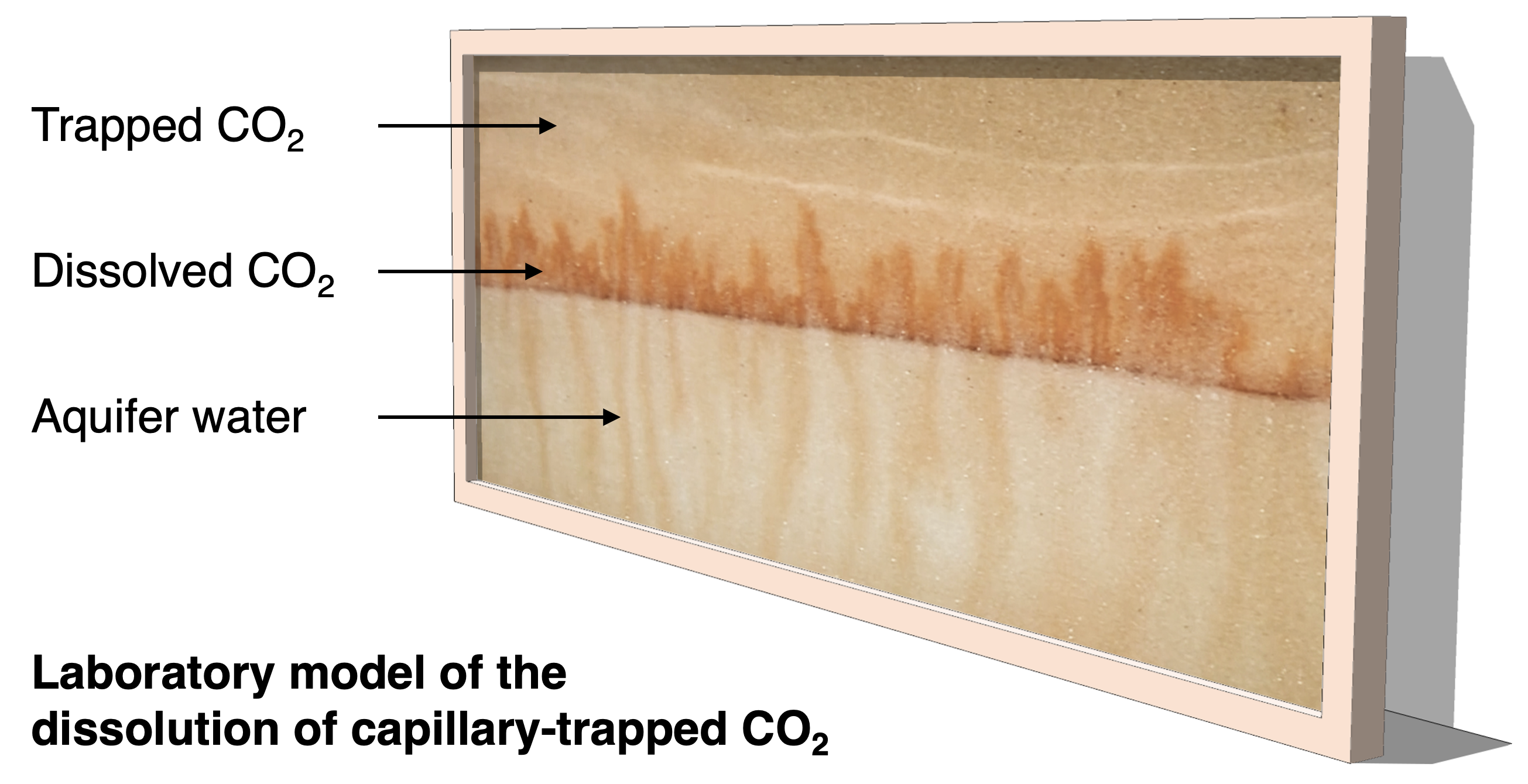
The experiment indicates that the pore water surrounding the trapped CO2 rapidly becomes saturated in CO2. Once saturated, the density of this water exceeds that of the background formation water. This leads to convective instability of the water in the aquifer, with fingers of dense, saturated water descending towards the base of the aquifer and fingers of buoyant, unsaturated water rising into the region with trapped CO2. This convective flow controls the dissolution of the CO2 in the aquifer.
We have developed a new model of the finger motion and the non-linear dissolution process. This model is in good accord with the experiments and predicts the maximum amount of trapped CO2 which can be dissolved in the aquifer, as well as the time required by the dissolution.
Follow the Topic
-
Communications Earth & Environment

An open access journal from Nature Portfolio that publishes high-quality research, reviews and commentary in the Earth, environmental and planetary sciences.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Archaeology & Environment
Publishing Model: Hybrid
Deadline: Mar 31, 2026
Drought
Publishing Model: Hybrid
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in