Copper(I)-catalyzed diastereo- and enantio-selective construction of optically pure exocyclic allenes
Published in Chemistry

Chiral allene moieties exist in about 150 natural products and a variety of functional synthetic compounds1-3. Due to the unique structural features and versatile reactivity of allenes, significant applications have been found not only in medicinal chemistry and material science, but also as important intermediates in synthetic transformations, and chiral ligands or catalysts in asymmetric catalysis. Among these identified allenic natural products, the exocyclic allenes constitute a major subclass, such as Neoxanthin, Grasshopper ketone, Citroside A, and fungal metabolite A82775C.
Substantial efforts are devoted to the construction of axially chiral allenes, however, the strategies to prepare chiral exocyclic allenes are still rare. Herein, we show an efficient strategy for the asymmetric synthesis of chiral exocyclic allenes with the simultaneous control of axial and central chirality through copper(I)-catalyzed asymmetric intramolecular reductive coupling of 1,3-enynes to cyclohexadienones. This tandem reaction exhibits good functional group compatibility and the corresponding optically pure exocyclic allenes bearing cis-hydrobenzofuran, cis-hydroindole, and cis-hydroindene frameworks, are obtained with high yields (up to 99% yield), excellent diastereoselectivities (generally >20:1 dr) and enantioselectivities (mostly >99% ee). Furthermore, a gram-scale experiment and several synthetic transformations of the chiral exocyclic allenes are also presented.
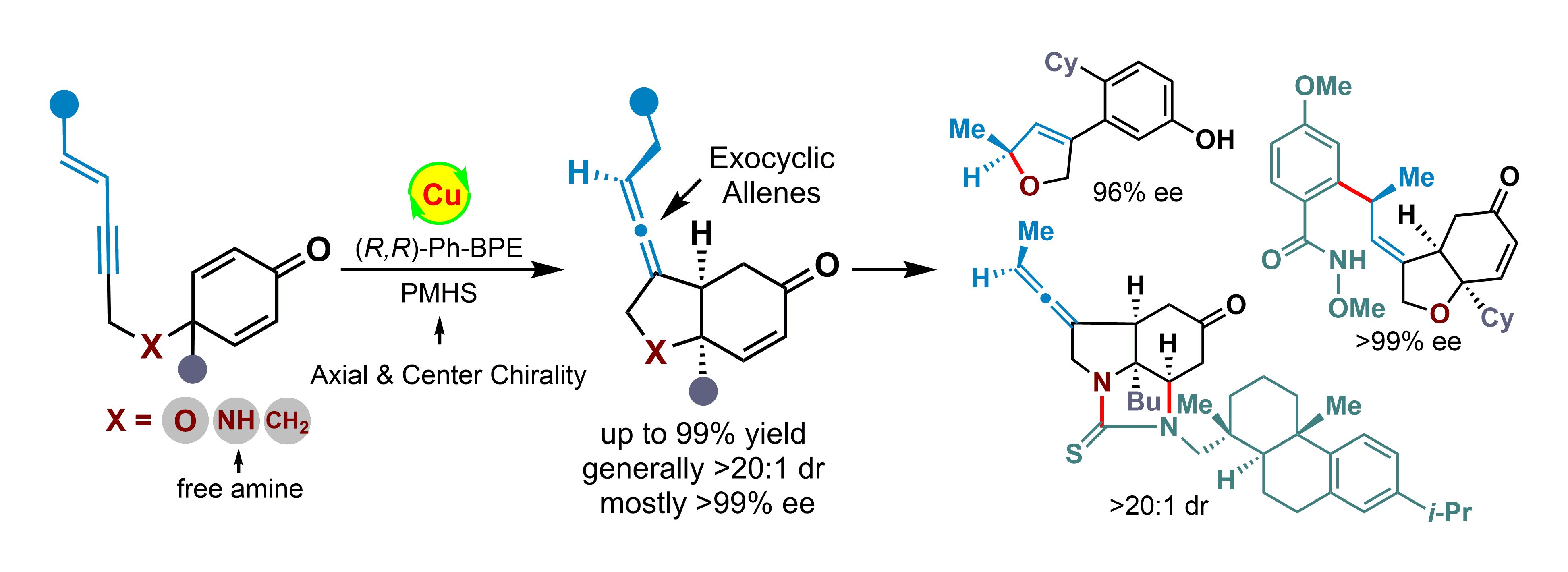
In a word, this work describes a copper-catalyzed asymmetric synthesis of exocyclic allenes by simultaneous control of axial and central chirality.
References:
- Krause, N. & Hashmi, A. S. K. Modern allene chemistry (Wiley, 2004).
- Hoffmann-Röder, A. & Krause, N. Synthesis and properties of allenic natural products and pharmaceuticals. Angew. Chem. Int. Ed. 43, 1196–1216 (2004).
- Yu, S. & Ma, S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 51, 3074–3112 (2012).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in