Could hamsters be a model for respiratory post-acute sequelae of SARS-CoV-2 ?
Published in Healthcare & Nursing

How did it start?
Everything began in December 2020, when we saw the lung lesions induced by experimental SARS-CoV-2 infection of a Syrian golden hamster for the first time. A week after the infection, the animals clear most of the virus and the histologic appearance of the lung is dominated by regenerative responses. This means that the surviving cells of the lung are dividing and migrating to replace the epithelial cells destroyed by the virus. In the hamster, the morphology of this process is very impressive, up to 50-60% of the lung sections, depending on the SARS-CoV-2 variant, are occupied by dense carpets of proliferating cells. In a healthy lung, alveolar spaces are empty and lined by very thin cells that allow gas exchange (AT1: alveolar pneumocytes type 1). In contrast, in the post-SARS-CoV-2 lung, alveoli are covered by plump, large and sometimes very bizarre-looking cells with huge or multiple nuclei. Some of the cells showed a morphology resembling pneumocytes type 2 (AT2) – the cell type is the main source for new alveolar cells (alveolar regeneration). Other regions in the lung were looking more like airway epithelium, the cell type covering bronchi and bronchioles, which is normally not present in the alveoli. The morphology and extent of that response is so massive and atypical, that it is partly resembling neoplastic cells. Two weeks after infection, at a time when the virus is long gone, this proliferation was still present in some areas, indicating that the original lung microscopical anatomy, and its physiology has not been restored yet. Additionally, the lungs show areas of fibrosis – an indicator of irreversible damage and scarring. These observations prompted many questions. What are these proliferating cells and where do they come from? Is this response normal? Will it resolve over time or result in lasting abnormalities? And - most importantly – does it have an impact on the clinical outcome of COVID-19?
How does a lung regenerate?
Many respiratory viruses damage the epithelial cells in the airways and the alveoli. The restoration of these structures depends on progenitor cells that can divide and differentiate into different cell types. The classical paradigm of lung regeneration states that airway epithelia are replenished by basal cells residing in bronchi and bronchioles, while alveolar AT1 cells are regenerated by transdifferentiating AT2 cells. This paradigm has been recently updated by the observation that, upon severe alveolar injury, multiple airway progenitor cells can mobilize to the alveoli and differentiate into AT2 and AT1 cells, aiding the regeneration processes (Figure 1).1,2,3,4
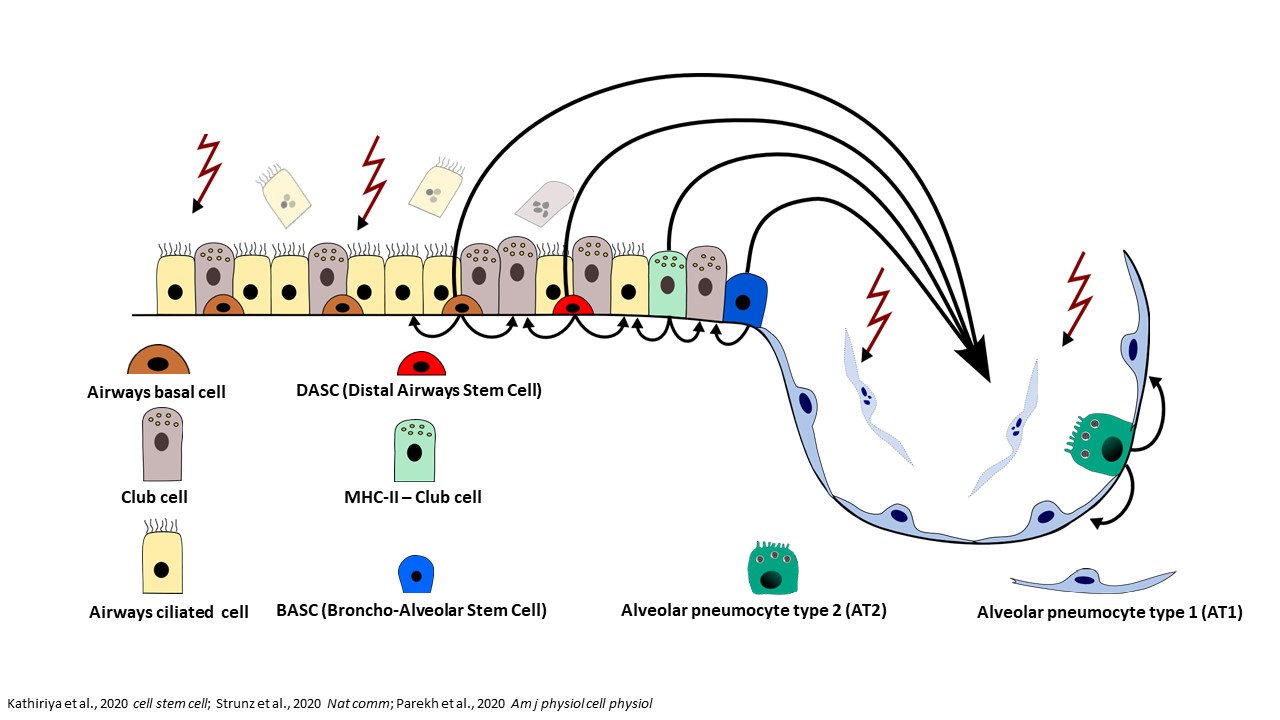
Another detail of alveolar regeneration that has been recently characterized is a distinct, intermediate cell state between AT2 and AT1 cells, the “alveolar differentiation intermediate” (ADI) cells. ADI cells occur transiently in physiological conditions of lung homeostasis and in regeneration after injury, but can accumulate and persist in certain diseases. The block in the ADI cell state can result in delayed lung regeneration and consequent prolonged impairment of gas-exchange. Persistence of ADI cells is also associated with development of lung fibrosis, which additionally compromises lung function (Figure 2).5,6,7,8

A contribution of airway progenitors to the alveolar regeneration processes and an accumulation of ADI cells has been described in fatal COVID-19 cases. 9, 10 It was suggested, that an impaired lung regeneration could be the reason for prolonged hypoxia in severe COVID-19, which keeps patients on ventilators longer than in other respiratory diseases. Later on, a new aspect of the COVID-19 arose. Patients surviving acute COVID-19 are at risk to develop the so called “long-COVID” or post-acute sequelae of SARS-CoV-2 (PASC).11,12,13 PASC occurs in 3-11.7% of infected individuals and is characterized by a variety of symptoms, such as fatigue, headache, cognitive dysfunction, altered smell and taste, shortness of breath, and dyspnea, occurring >12 weeks after acute virus infection.14,15 Of note, among patients with severe disease requiring hospitalization, respiratory symptoms are reported with a much higher frequency. When PASC was starting to gain more attention, the scientific community could only guess what the mechanisms of this phenomenon are. Among other hypotheses, it was suggested that impaired lung regeneration and fibrosis could be responsible for long-term symptoms. In order to investigate this further, a reliable animal model was needed.
Several animal models have been used to model various aspects of COVID-19, but until very recently, the majority of studies focused on the acute infection phase and little attention was given to the mechanisms of lung regeneration. One of the most used model species is the Syrian golden hamster, a species that has not been excessively used in the lab prior to COVID-19. When we started this project, it was unknown whether lung regeneration in the hamster can be compared to what happens in human patients. Being veterinary pathologists, we know that one species can have a totally different response to injury than another. Therefore, we decided to characterize the regenerative response in the hamster to evaluate its value for modeling long-term consequences of SARS-CoV-2 infection. We hypothesized that ADI cells and airway progenitors contribute to the lung regeneration in the model and that the atypical cells we saw histologically could be ADI cells blocked in the intermediate state.
What did we show?
In this work, we show further insights in the regenerative processes following SARS-CoV-2 infection. To achieve this, we characterize the proliferating epithelial cells within the lungs of infected hamsters in the acute and sub-acute phase of the infection until 14 days post infection. Our study shows that CK8+ADI cells and multipotent CK14+ airway basal cells participate in alveolar regeneration and that persistence of ADI cells at 14 dpi is associated with fibrosis in SARS-CoV-2 infected hamsters. In addition, our study provides a hamster-specific marker gene lists for different alveolar cell populations, including AT1, ADI and AT2 cells. Altogether, the results provide important information on a translational COVID-19 model, which is crucial for its application in future research addressing pathomechanisms of PASC and for testing of prophylactic and therapeutic approaches for this syndrome.
What are the impact and implications of our study?
Our study offers a detailed characterization of cell populations composing the pulmonary epithelial regenerative response in the hamster and thus provides highly needed information about this important translational COVID-19 model. Studies investigating vaccines and novel therapeutical options using the model should consider to evaluate these parameters of lung regeneration.
Since post-COVID-19 pathological lesions show overlap with other diseases featuring diffuse alveolar damage (e.g. influenza infection) and lung fibrosis, the model can be used for broader implications. We show that ADI cells and airway-derived progenitors participate in alveolar regeneration in this species and provide evidence of ongoing regeneration post virus-clearance. Thus, hamsters are a suitable model to investigate the relevance of these changes and their actual contribution to PASC symptoms. For this reason, the established model can be used for future studies using longer timepoints and testing treatment options against PASC.
If you are interested and you would like to know more about lung regeneration in the hamster model for COVID-19, please feel free to contact us:
wolfgang.baumgaertner@tiho-hannover.de
federico.armando@tiho-hannover.de
References
- Liu, Q. et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 51, 728-738, doi:10.1038/s41588-019-0346-6 (2019).
- Liu, K. et al. Bi-directional differentiation of single bronchioalveolar stem cells during lung repair. Cell Discov 6, 1, doi:10.1038/s41421-019-0132-8 (2020).
- Kathiriya, J. J., Brumwell, A. N., Jackson, J. R., Tang, X. & Chapman, H. A. Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration. Cell Stem Cell 26, 346-358 e344, doi:10.1016/j.stem.2019.12.014 (2020).
- Barkauskas, C. E. A Specialized Few Among Many: Identification of a Novel Lung Epithelial Stem Cell Population. Cell Stem Cell 26, 295-296, doi:10.1016/j.stem.2020.02.010 (2020).
- Jiang, P. et al. Ineffectual Type 2-to-Type 1 Alveolar Epithelial Cell Differentiation in Idiopathic Pulmonary Fibrosis: Persistence of the KRT8(hi) Transitional State. Am J Respir Crit Care Med 201, 1443-1447, doi:10.1164/rccm.201909-1726LE (2020).
- Strunz, M. et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11, 3559, doi:10.1038/s41467-020-17358-3 (2020).
- Riemondy, K. A. et al. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight 5, doi:10.1172/jci.insight.123637 (2019).
- Kobayashi, Y. et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Cell Biol. 22, 934-946, doi:10.1038/s41556-020-0542-8 (2020).
- Melms, J. C. et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 595, 114-119, doi:10.1038/s41586-021-03569-1 (2021).
- Zhao, Z. et al. Single-cell analysis identified lung progenitor cells in COVID-19 patients. Cell Prolif 53, e12931, doi:10.1111/cpr.12931 (2020).
- A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021, <https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1> (2021).
- Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601-615, doi:10.1038/s41591-021-01283-z (2021).
- Castanares-Zapatero, D. et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 54, 1473-1487, doi:10.1080/07853890.2022.2076901 (2022).
- Sudre, C. H. et al. Attributes and predictors of long COVID. Nat Med 27, 626-631, doi:10.1038/s41591-021-01292-y (2021).
- Ayoubkhani, D. P. & Gaughan, C. Technical article: Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. <https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021> (2021).
- Staudt, A. et al. Associations of Post-Acute COVID syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur J Intern Med 95, 50-60, doi:10.1016/j.ejim.2021.10.031 (2022).
- Fernandez-de-Las-Penas, C., Martin-Guerrero, J. D., Cancela-Cilleruelo, I., Moro-Lopez-Menchero, P. & Pellicer-Valero, O. J. Exploring the recovery curve for long-term post-COVID dyspnea and fatigue. Eur J Intern Med 101, 120-123, doi:10.1016/j.ejim.2022.03.036 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in