Creating synthetic receptors: An idea born at lunch
Published in Bioengineering & Biotechnology, Microbiology, and Protocols & Methods

The initial idea for this project was conceived, as surely is true for countless projects, from a discussion at lunch. It was your typical sunny day at Stanford in the summer of 2021 and several of the (eventual) co-authors and I were sitting around a table at an outdoor cafe (Fig. 1). I remember discussing our recent success modeling mutations in the erythropoietin receptor (EPOR) that had been identified in an Olympic cross-country skier. In this work, which was eventually published as its own story1, we used CRISPR to pair this naturally occurring EPOR mutation with curative edits for sickle cell disease and beta-thalassemia to amplify production of functional red blood cells (RBCs).

Fig. 1: The outdoor cafe where the idea for this project was originally conceived.
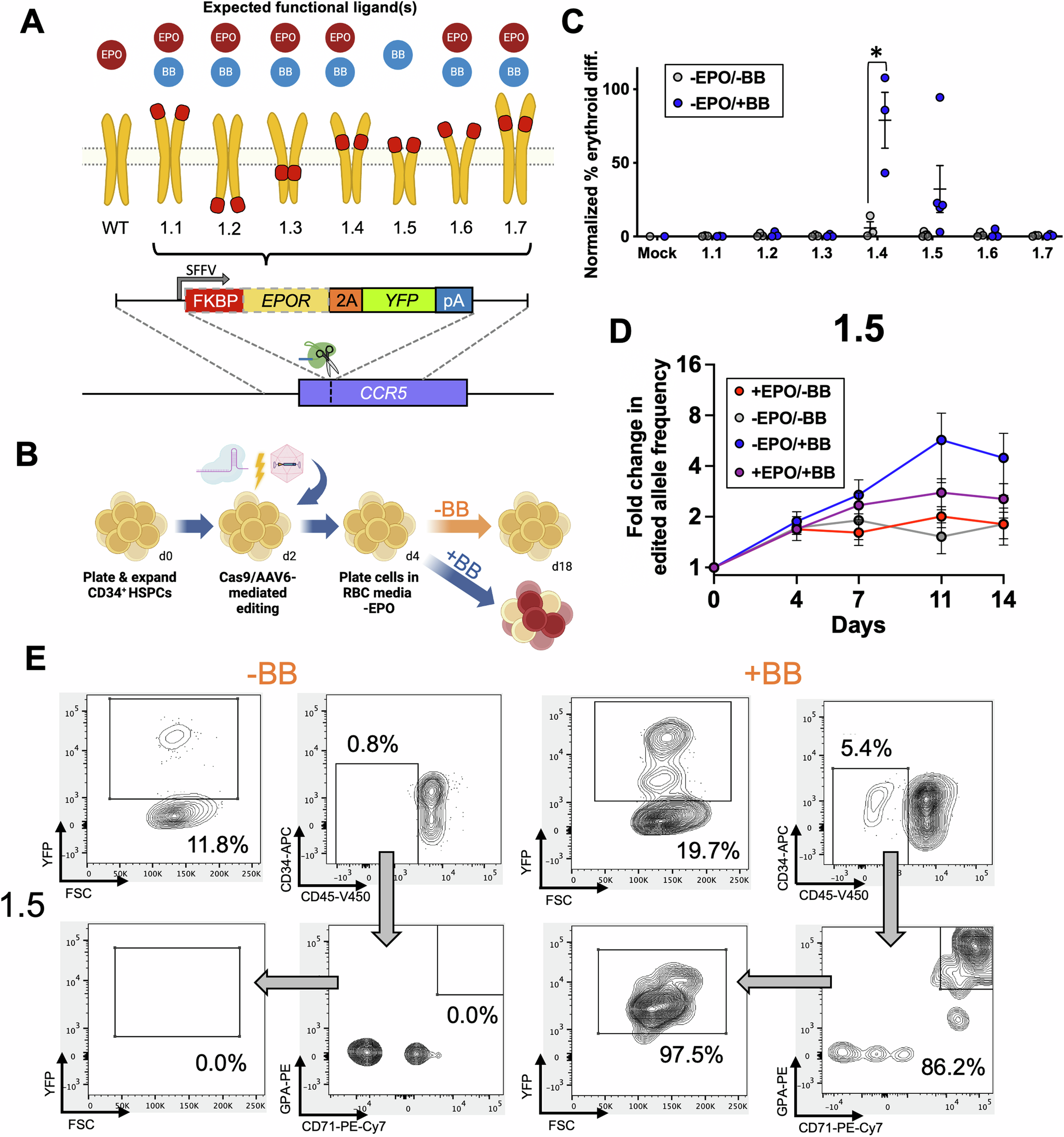
Sitting around the table, we were musing about what else could be done with the EPOR, especially since we had found it so easy to manipulate and model the receptor in human hematopoietic stem cells (HSCs) in vitro. We began discussing how erythropoietin (EPO) activates downstream signaling by bringing together two units of EPOR, analogous to a key-in-a-lock. This led to one of the co-authors musing whether, if it was really that simple, then could we repurpose dimerization domains to make a small molecule-inducible EPOR? It was an interesting idea, especially since we had successfully deployed these domains in a prior project in the form of inducible safety switches2.
While most conversations would end there, several of us couldn’t let it go. We continued the conversation on the walk back to the lab and into the next day. However, this seemed like one of those ideas where it would either work great or not at all. Therefore, complemented by a thorough literature search and some additional consideration, we came up with a fail-fast approach. This would allow nature to tell us very quickly whether the idea was going to work. We designed a small initial library of candidate synthetic EPOR designs and resolved only to proceed if one of the designs showed signs that it was working as expected.
Fast forward a month or two later, receptor designs had been built and were ready for testing. To our delight, we found that 2 out of the initial 7 designs worked, enabling us to generate RBCs by stimulating cells with small molecule instead of EPO. We knew from this moment that we had a project, and that with even a little traction we had a number of ideas for how to enhance and fine-tune our system.
This was not the end of the story. Because in June 2022, less than a year after our idea at lunch, I transitioned from my role as a junior faculty member at Stanford to beginning my own lab at UCSF. By this time, we had further optimized our synthetic EPOR (synEPOR) designs, but now were met with a challenge that had nothing to do with the science. If you have ever tried to wrap up a project after leaving a lab, then you know what I’m talking about!
This project then became more a tale of open communication, mentorship spanning institutions, and a true team effort. Because I viewed this project as representing a new direction for my fledgling lab—going deeper into synthetic biology and cell engineering—I was very invested in keeping the momentum up. This meant many drives between Stanford and UCSF, many different sets of hands on pipettes at both institutions, and a hundred zoom calls.
At this point, we had demonstrated that we could exquisitely recapitulate EPOR signaling using our synEPORs. However, it became clear that we had a very interesting tool in search of its ideal use case. This is when the project was impacted by cross-pollination, highlighting the benefits of moving your science to a new institution. As a young PI, I found myself in a new scientific environment and my research viewed from a different perspective by the many talented people at UCSF. In an early discussion with one of my new colleagues, I showed some of the data from our Olympic skier and synEPOR projects. My colleague then pulled up the first presentation he ever gave at UCSF, which was on the potential to manufacture RBCs in bioreactors to address blood shortages. He said that he thought the genome engineering we were doing could help solve some of the bottlenecks to scalable ex vivo RBC production. In particular, he directed me to several reviews that highlighted the cost of cell culture media and recombinant cytokines, particularly EPO, being a major barrier to cost-effective RBC manufacturing3.
While we had originally considered our tool to be useful to allow tunable control of HSCs post-transplant for treatment of sickle cell disease or thalassemia, this concept would require extensive and complicated modeling in animals and may be less effective than simply introducing the Olympic skier mutation. This conversation opened my eyes to another application that would be more straightforward and potentially more impactful, as RBC transfusion impacts virtually every American family at some point. From this moment on, we had clarity on what experiments needed to be done to close out our project.
I imagine for those reading this Behind the Paper essay, our story may sound familiar. It may remind you of the ideas that you have discussed with friends over lunch, some of which you may have pursued, but many you did not. Throughout my career, this is how I have seen the best and most organic science materialize. It is not just a brilliant idea and the rest is history. Instead, the most impactful science often takes shape gradually, refined through collaboration and the diverse expertise of those involved. Perhaps this story serves as a reminder that some of the most promising ideas start as casual conversations—and are worth pursuing.
References:
- Luna, S.E., Camarena, J., Hampton, J.P., Majeti, K.R., Charlesworth, C.T., Soupene, E., Selvaraj, S., Jia, K., Sheehan, V.A., Cromer, M.K., et al. (2024). Enhancement of erythropoietic output by Cas9-mediated insertion of a natural variant in haematopoietic stem and progenitor cells. Nat. Biomed. Eng. 10.1038/s41551-024-01222-6.
- Martin, R.M., Fowler, J.L., Cromer, M.K., Lesch, B.J., Ponce, E., Uchida, N., Nishimura, T., Porteus, M.H., and Loh, K.M. (2020). Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat. Commun. 11, 2713. 10.1038/s41467-020-16455-7.
- Timmins, N.E., and Nielsen, L.K. (2009). Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 27, 415–422. 10.1016/j.tibtech.2009.03.008.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in