
At a 1984 press conference announcing the identification of HIV as the cause of AIDS, then-secretary of Health and Human Services Margaret Heckler told reporters, “We hope to have a vaccine ready for testing in about two years.” Thirty-nine years and billions of dollars later, we’re still waiting. HIV has three key characteristics that make it a unique challenge for vaccine development: antigenic variation, metastability, and an unusually dense glycan shield.
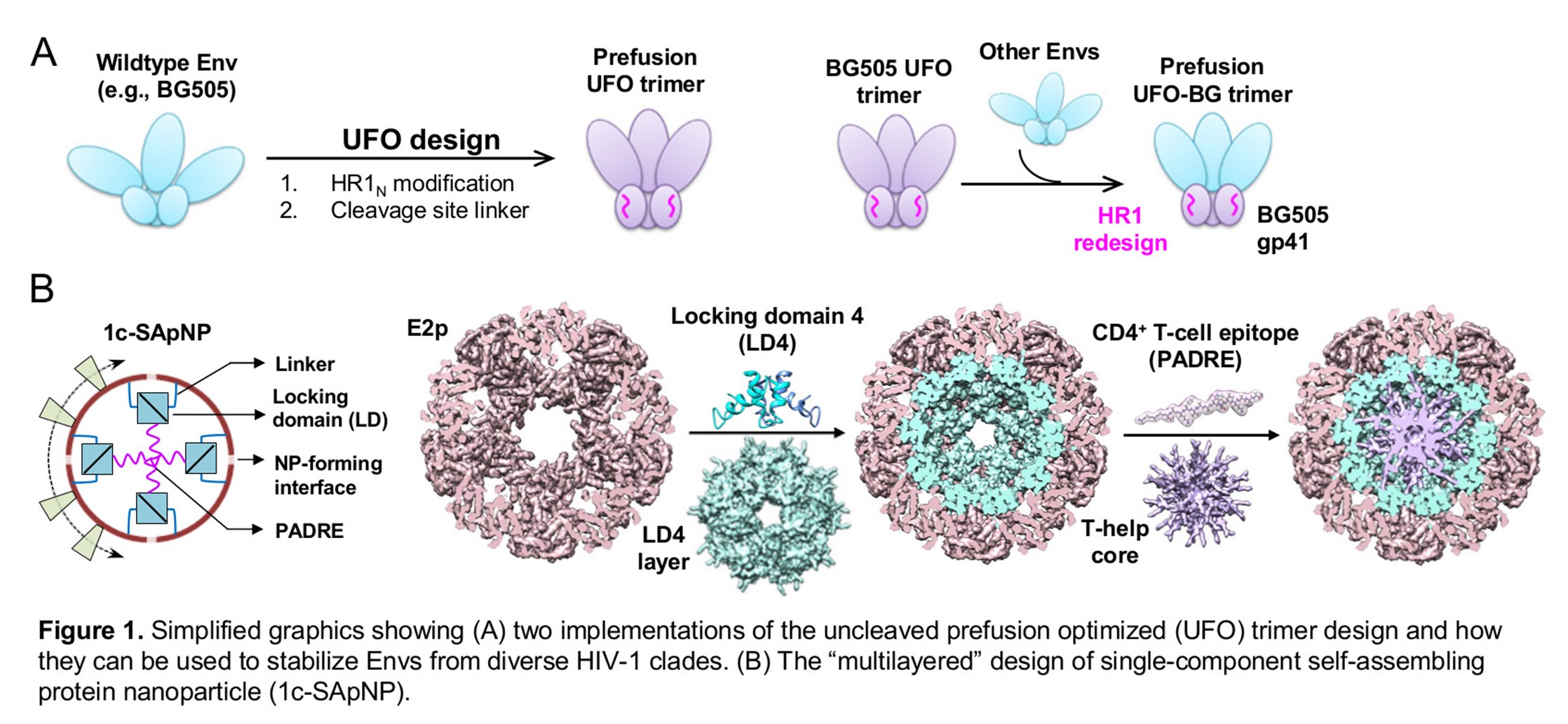
Because of HIV’s high levels of antigenic variation, a major focus of vaccine design has been to generate broadly neutralizing antibodies (bNAbs) which recognize a functionally conserved viral target – one that can’t easily be changed without weakening the virus’ ability to infect cells. All bNAbs against HIV target a trimeric protein spike, called Env, found on the viral surface. To be effective, bNAbs must bind to Env before HIV fuses with the host cell. However, HIV Env is metastable, meaning that it tends to spontaneously and irreversibly change from a prefusion form to a very different postfusion form. Previous trimer designs used strong molecular bonds to hold the trimer in a prefusion state; we designed our own trimer by disabling the molecular “spring” that pushes the trimer open in the first place. Because our uncleaved prefusion optimized (UFO) trimer design eliminates the root cause of metastability instead of fighting against it, it requires fewer alterations to the wild-type Env sequence than existing designs and is easier to apply to Envs from many different HIV strains.
But the trimers by themselves didn’t elicit a strong enough immune response. The next challenge was to present stabilized Env trimers in such a way that the immune system would recognize them as a threat. One way to make antigens “look” more like a real pathogen to the immune system is to display them on a nanoparticle that’s roughly the size and shape of an actual virus. We designed a single-component, self-assembling protein nanoparticle (1c-SApNP) by fusing the genetic sequence for our UFO trimer to the sequence for an engineered bacterial enzyme (E2p or I3-01v9) that self-assembles into a spherical, multilayered nanoparticle. This allows the viral antigen and nanoparticle scaffold to be produced all in one piece, making the resulting nanoparticle easier to produce and more stable than ones in which the antigens and nanoparticles are made from separate pieces and then “glued” together. The 1c-SApNP vaccines were much better recognized by known bNAbs than trimers in vitro and can stay in lymph nodes for weeks to stimulate the immune responses, but their responses in animals were not as strong as expected. We still had one more challenge to overcome: the glycan shield.
Glycosylation is a common post-transcriptional modification in which long, branched sugar molecules called glycans are attached to a protein. Because viruses hijack the host cell’s machinery to make their own proteins, those viral proteins are glycosylated like the cell’s own proteins, which helps to hide them from the immune system. In the case of the HIV Env protein, the glycans are so thick that they form a dense glycan shield that prevents the immune system from recognizing the protein structures underneath, like hair covering your face. But glycosylation can also contribute to the immune response: glycans can help stabilize bNAb-antigen binding, and quite a few bNAbs bind to the glycans themselves. Some studies have suggested that HIV antigens need glycans to travel to the lymph nodes, where they can encounter T cells and B cells needed to generate a vaccine response. The Jekyll-and-Hyde nature of glycosylation has been a major challenge for HIV vaccine research, and so far, there’s no consensus on what an ideal vaccine should look like. We spent years testing different glycan modifications, trying to find the sweet spot that would uncover Env’s “face” without losing the beneficial glycan functions. Finally, we decided to try something drastic: since subtle glycan modifications weren’t working, we gave Env a “buzz cut” by treating our vaccine particles with endoglycosidase H, an enzyme that trims glycans down to a single N-acetylglucosamine.
Glycan trimming turned out to be a crucial piece to the puzzle, exposing important bNAb binding sites while retaining Env binding to bNAbs that need glycans in their interactions. More importantly, glycan-trimmed trimers on SApNPs were much more effective at generating tier 2 virus (isolated from patients) neutralizing antibody responses in mice. We decided to test our new glycan-trimmed (GT) vaccines in nonhuman primates (NHPs) as well. Since we had a limited number of rhesus macaques, we weren’t able to do a side-by-side comparison in a single experiment. Still, the results were quite dramatic: in the first NHP experiment performed with WT SApNPs, only 1 macaque out of 12 had a barely detectable response. In the second NHP experiment, 7 out of 16 produced autologous (vaccine-matched) neutralizing antibodies as long as the vaccine was “glycan trimmed”.
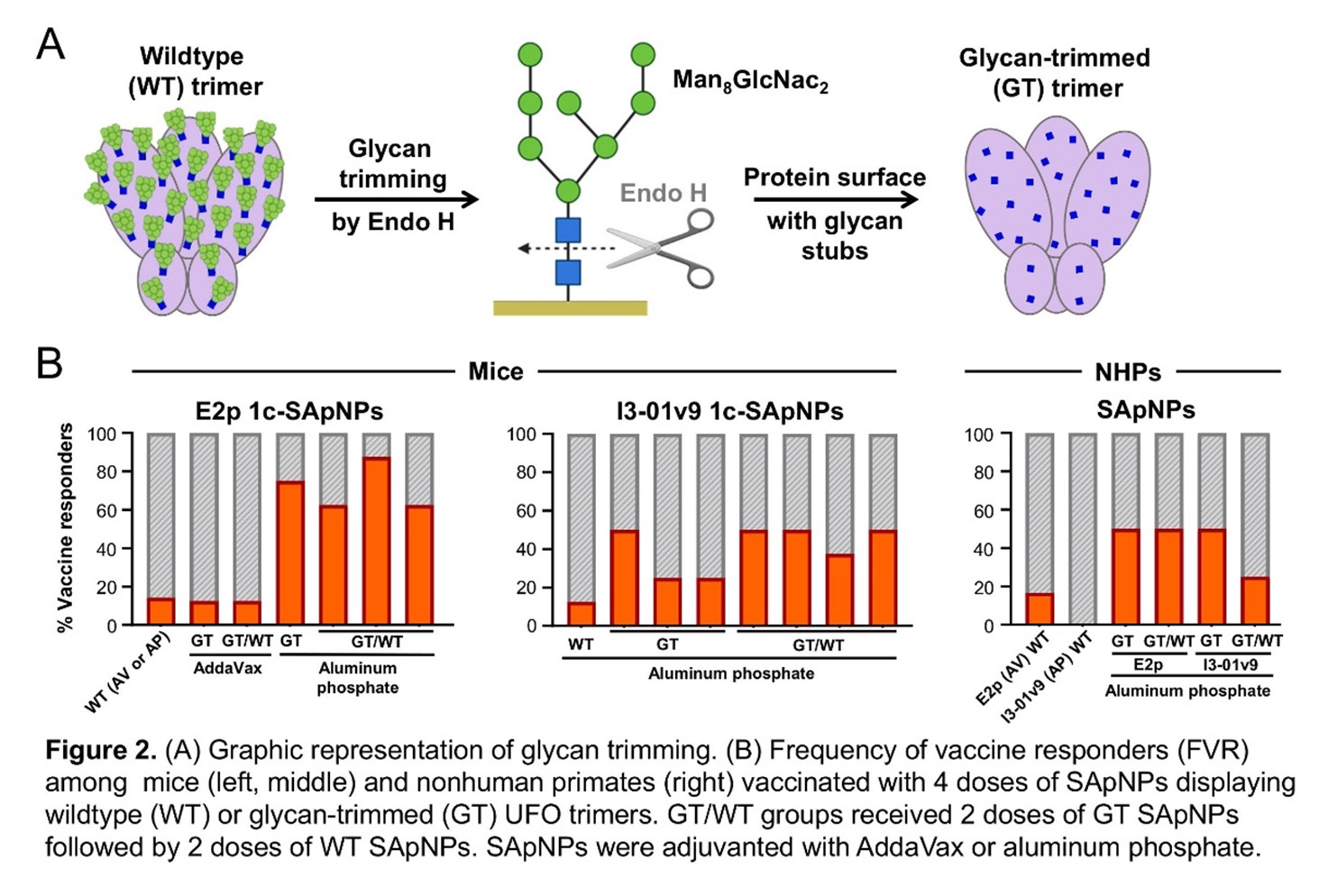
This is an exciting breakthrough: now we know that glycan trimming can significantly improve the frequency of vaccine responders (FVR) – the percentage of animals in a vaccine group that generate a robust autologous virus-neutralizing response. But we want our vaccine response to be more potent and broader, so we are still looking for the last “missing piece” to the HIV vaccine puzzle. We are now testing our SApNP vaccines with different adjuvants – compounds that can enhance the immune responses to a vaccine. For example, we found that the E2p SApNPs are more effective paired with aluminum phosphate rather than AddaVax. We are going to test some potent adjuvants recently approved for human use and work with some of the best chemists to develop new ones. Another approach we’re exploring is making SApNPs with Envs from different clades – families of closely related HIV strains. It may be easier to generate antibodies that will neutralize many strains within the same clade than cross-clade bNAbs, and if we can generate NAbs against just three clades – A, B, and C – then we could prevent approximately 86% of new HIV infections.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in