Database of Photocatalytic Dehydrogenation Reactions
Published in Materials and Research Data
Explore the Research
 sciencedirect.com
sciencedirect.com
Just a moment...
Skip to main content
Dehydrogenation of an organic compound – conversion of a compound into its unsaturated counterpart and hydrogen (H2) – is endothermic (∆H0 > 0) and endergonic (∆G0 > 0) reaction, in most cases. Therefore, the choice of photocatalysis as a method to drive this reaction is fully justified by the necessity not only to overcome reaction energy barriers, but to lift a chemical system to a higher energy level.
Fellow chemists who create new photocatalysts and publish their results in peer-reviewed journals would, probably, agree that one of the most popular referees’ questions is: “What is the performance of your photocatalyst in comparison with others?” As the indicator of this aspect, practically every article that deals with the preparation of a new photocatalyst is supported by a table or a plot, which summarizes the most active of them, and their key performance indicators, such as quantum yield, and productivity.
Among the research directions of my group is selective photocatalytic dehydrogenation of organic compounds. Earlier, I wrote about the conversion of ethanol into H2 and 1,1-diethoxyethane performed using a photocatalyst made of Pt nanoparticles and poly(heptazine imide) in a flat-panel 550-cm2 photoreactor using real sunlight. Owing to high efficiency of the photocatalyst (AQY is 73% at 410 nm), this photoreactor generates 150 mL of H2 per hour. To rank properly the photocatalysts that are being developed in my group in comparison with those reported earlier and to give the community of researchers a tool for the same purpose, I created a Database of Photocatalytic Dehydrogenation Reactions. It is available via the link (CC BY-NC 4.0).
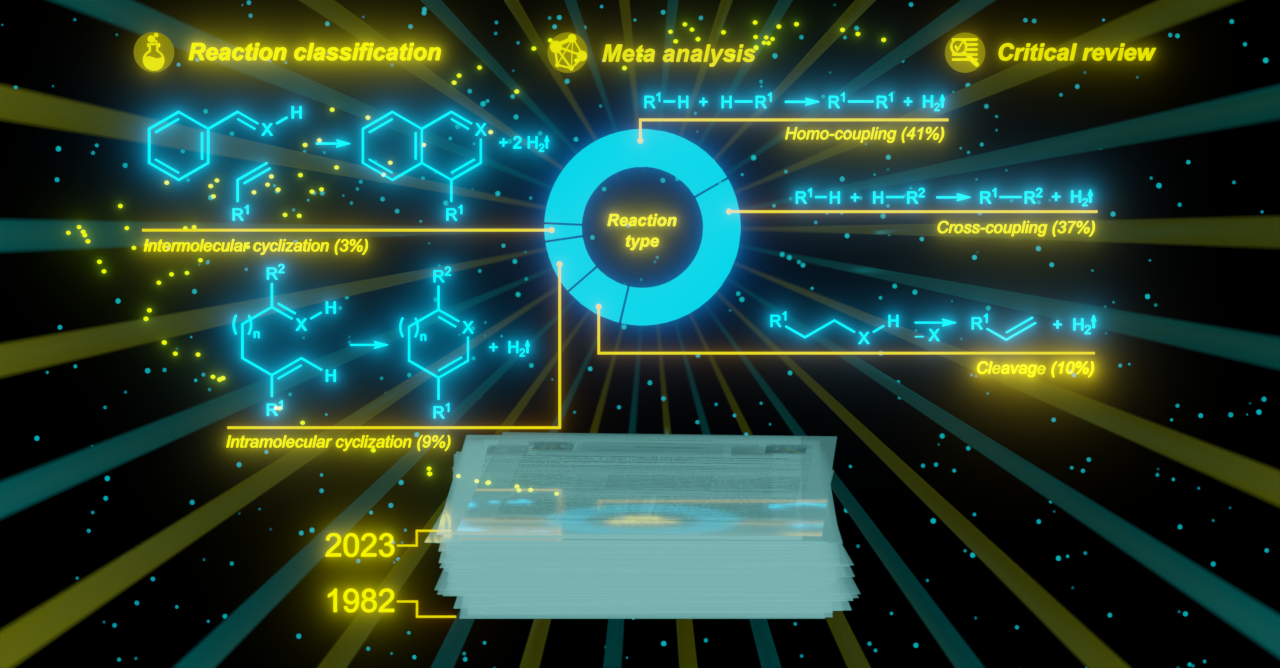
It exists as a table of data, 236 entries x 100 classification parameters and key performance indicators. The most important of them are:
- Reaction classification according to the type of the formed bond, such as C–C, C–N, C–O, C–P, etc.
- Reaction classification based on the hybridization of carbon atoms involved in the formation of the chemical bond, such as sp3–, sp2–, sp–.
- Reaction classification based on the kind of chemical transformation, such as cross-coupling (synthesis of a product from two or more reagents) vs. homo-coupling (di- and oligomerization of the reagent), intramolecular cyclization vs. intermolecular cyclization, extent of reagent dehydrogenation (desaturation of the reactant vs. aromatization), etc.
- Photocatalyst classification based on its type – homogeneous vs. heterogeneous.
- Photocatalyst classification based on its chemical structure, such as molecular transition metal complexes, semiconductors.
- Classification of H2-evolution catalyst based on its type – homogeneous vs. heterogeneous.
- Classification of H2-evolution catalyst based on its chemical structure, such as molecular transition metal complexes, transition metal (nano)particles.
- Assembly of the photocatalyst and H2-evolution catalyst, such as reduction of H2-evolution catalyst precursor at the photocatalyst, solvothermal growth of H2-evolution catalyst at the photocatalyst, etc.
- Type, chemical structure and amount of the organocatalyst, hydrogen atom transfer (HAT) catalyst and/or additive(s).
- Quantum yield of the reaction.
- Classification based on the used reactant – its chemical structure, amount (moles, grams), concentration.
- Classification based on the major organic product – its chemical structure, yield and yield rate (in mol[product] g[catalyst]-1 h-1).
- Classification based on the amount of generated hydrogen – yield and yield rate of H2.
Using a spreadsheet editor, it is straightforward to identify the most performing photocatalyst in a specific reaction. Consider, for example, dehydrogenation of benzyl alcohol and synthesis of benzaldehyde – one of the most studied reaction in this area of research.
The highest yield rate of benzaldehyde, 183 mmol g-1 h-1, was reported by Dominic Tilgner and colleagues in Australian Journal of Chemistry 2019, 72(10), 842-847.
On the other hand, one could argue that the yield rate does not represent the photocatalyst, because this parameter depends on reaction conditions, in particular photon flux. Indeed, all the photocatalytic data collected in the database were acquired under not exactly the same conditions. Sorting the data based on the magnitude of the apparent quantum yield (AQY) reveals the work authored by Kazuya Imamura and colleagues in Applied Catalysis A 2013, 450, 28-33. They achieved AQY of 70% at 366 nm in dehydrogenation of benzyl alcohol.
Liberation of H2 from organic compounds, such as primary aliphatic alcohol can yield a series of products, in particular, aldehyde, 1,1-dialkoxyalkane and diol. Factors that influence selectivity of organic compounds dehydrogenation are discussed in the review article that we published in Chinese Journal of Catalysis.
Does the database and the review misses your article? Let me know by posting it in the comments below. The research field is developing rapidly. Now, we are working on the next edition of the database.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in