Pushing solar fuels to the next level
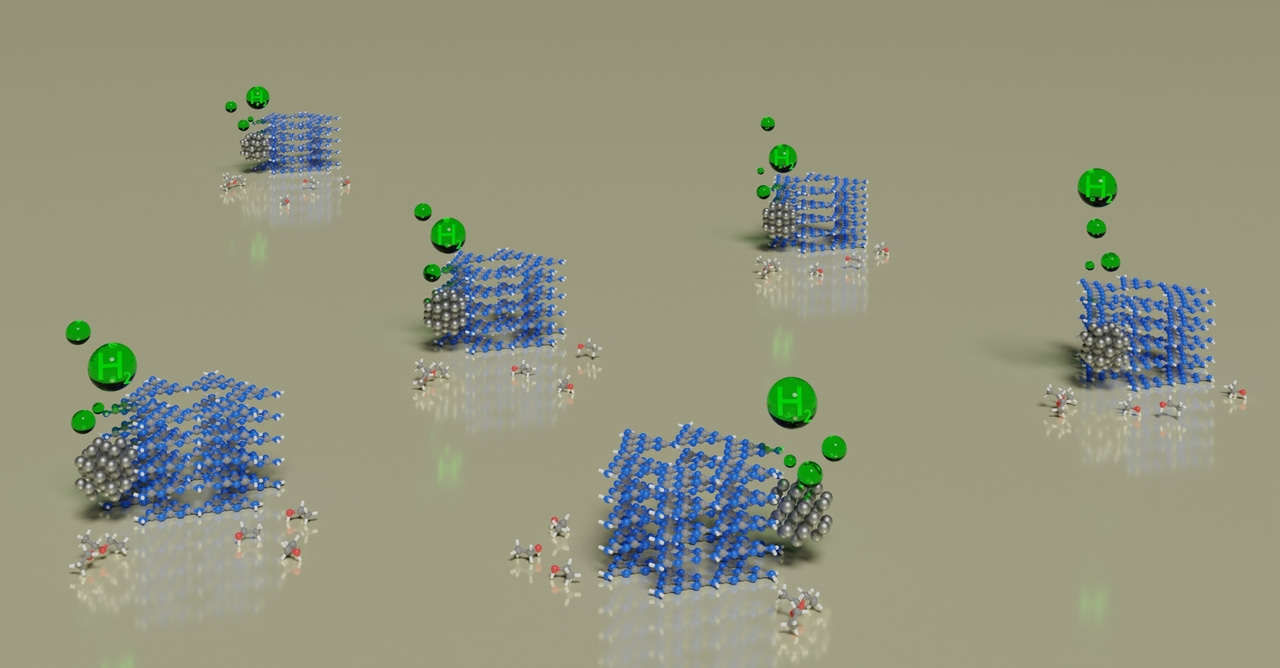
Generation of hydrogen (H2, "solar fuel") by sustainable methods is one of the strategic topics of the European Union. Therefore substantial resources are invested into creating new materials, method and processes, which can deliver this chemical.
In the project "GreenH2 production from water and bioalcohols by full solar spectrum in a flow reactor" (abbreviated as GH2 and funded under the European Innovation Council), one of our goals is to produce of H2 from bioalcohols with quantum efficiency >60%. Simultaneously with H2 we aim to obtain a product of a bioalcohol dehydrogenation with selectivity >90%. In case of bioethanol, such a product is 1,1-diethoxyethane. This molecule is used as a solvent, but has a potential as a fuel-additive.
Dr. Vitaliy Shvalagin has been working on the implementation of the abovementioned goals. He designed and prepared a photocatalyst, which is based on graphitic carbon nitride - poly(heptazine imide).
.png)
Readers who follow developments in the field of solar fuels have probably noticed similarities of our setup and a flat panel reactor developed by Prof. Kazunari Domen and colleagues. Indeed, we were inspired by their design. After all, immobilizing a photocatalyst on a flat surface is the most rational and straightforward photoreactor design when using natural sunlight.
This part of the GH2 project was completed in a cooperation with ETH Zurich, Hong Kong University and Tsinghua University. The results are now published in ACS Catalysis.
Before joining my group at the Max Planck Institute of Colloids and Interfaces in Potsdam, Germany, Vitaliy has been working as a senior research associate at the Institute of Physical Chemistry, National Academy of Sciences of Ukraine - a stable job and an advanced carrier stage within Ukrainian scientific community. After Russia's full-scale invasion of Ukraine, on 24th of February 2022, Vitaliy and his family had to leave Kyiv. Unplanned relocation and a new environment were the challenges they faced.
This project is not just about photocatalysis and hydrogen generation; it is a testament to the researcher’s determination in the face of adversity. Truly per aspera ad astra.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in