Deciphering the Heterogeneity and Lineage Plasticity of Prostate Cancer Using High-Throughput Data: Broader, Finer and Objective
Published in Cancer and Protocols & Methods
-
Why this study is important
Nearly all early-stage prostate cancer cases are dependent on androgen receptor (AR) signaling, making AR-targeted therapy a cornerstone of prostate cancer management. Unlike other cancer types where resistance to targeted treatment is primarily due to mutations in the drug target, prostate cancer can evade targeted treatment through lineage-switching. In this process, prostate cancer cells bypass their dependency on AR signaling by acquiring alternative phenotypes, with the neuroendocrine (NE) phenotype being the most observed. Although rare, AR-/NE- phenotypes have also been reported. Additionally, prostate cancer cells with different phenotypes can be observed within a single tumor, and their composition varies during cancer progression and treatment resistance. There is an urgent need for molecular landscaping studies to describe the different prostate cancer phenotypes and develop novel therapeutic targets.
-
How this research contributes to the field-directly
In our recently published paper, summarized in Figure 1, we performed a comprehensive and rigorous multi-omics study on prostate cancer samples from both humans and mice. We generated two prostate cancer single-cell datasets named HuPSA (human) and MoPSA (mouse) to describe cancer and stromal cell populations. Regarding cancer heterogeneity, we identified various AR+ prostate adenocarcinoma populations, neuroendocrine prostate cancer population, and, more importantly, two novel AR-/NE- populations named KRT7 and progenitor-like. We validated the existence of these novel populations using bulk RNAseq and immunohistochemistry. These identifications provided new diagnostic markers and laid the foundation for future mechanistic studies on these alternative phenotypes to develop new treatment strategies.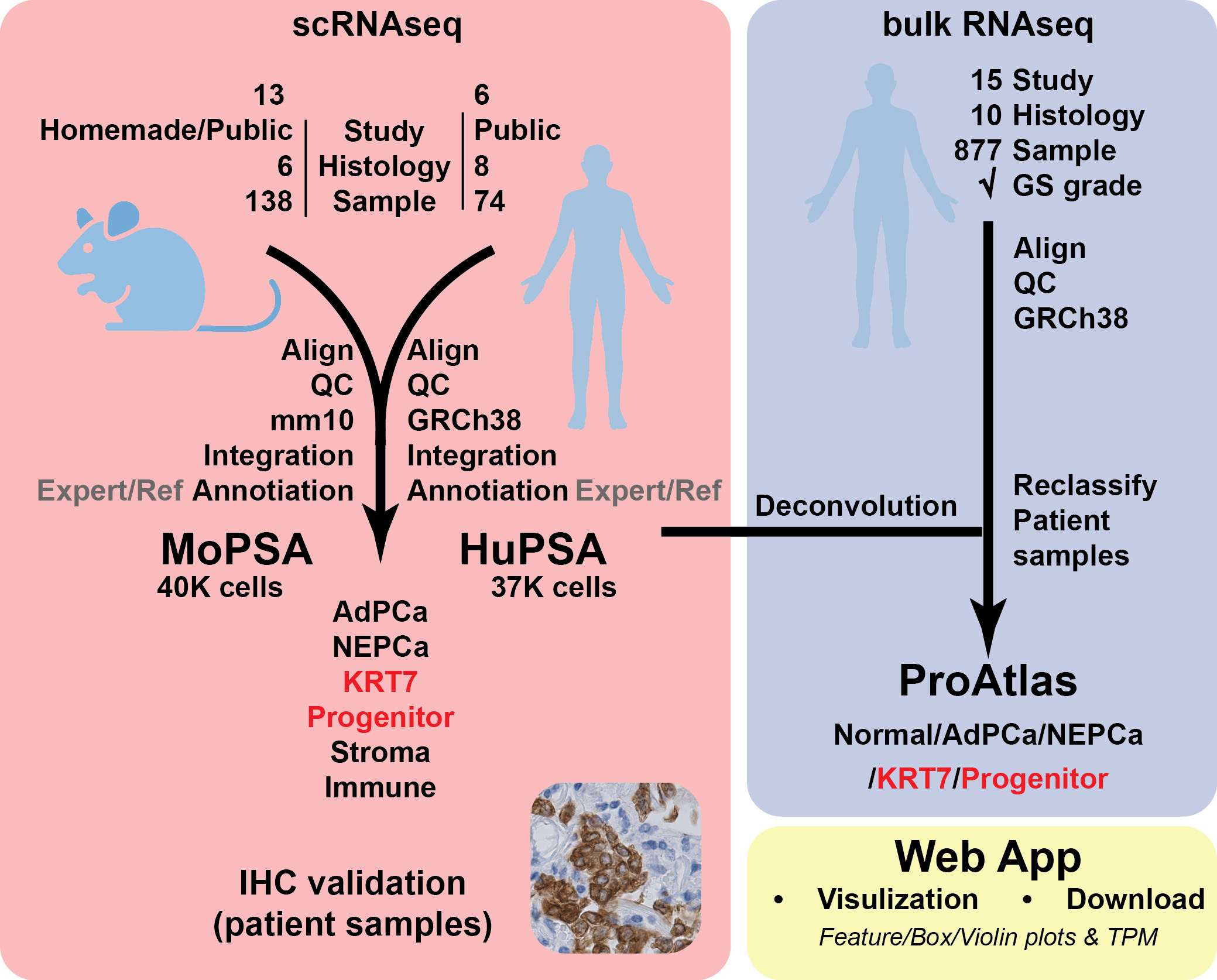
-
How this research contributes to the field-indirectly
While drafting the manuscript, we realized that the comprehensive datasets generated for our research are also valuable to others. Our research analyzed only a handful of gene expressions, but the remaining data could be a hidden gem for other researchers who are insightful and smart but may lack bioinformatics skills. To facilitate this, we launched the HuPSA&MoPSA online website, where researchers can freely browse, visualize, and download the three datasets we generated. We hope that other researchers can identify even more interesting findings using our tool.
Besides the current website, we have developed other bioinformatics websites with different focuses, as summarized in Tabel 1 below:
|
Name |
Purpose |
URL |
|
HuPSA&MoPSA |
Single-cell prostate cancer transcriptome |
|
|
ProAtlas |
Bulk prostate cancer transcriptome |
|
|
CTPC |
Prostate cancer cell line transcriptome |
|
|
PCTA |
Pan-cancer cell line transcriptome |
|
|
LNCaP-ADT |
LNCaP multi-omics |
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
Tumor-type-agnostic biomarkers and treatments in oncology
Publishing Model: Open Access
Deadline: Mar 05, 2026
Emerging adjuvant and neo-adjuvant treatment approaches in solid tumors
Publishing Model: Open Access
Deadline: Mar 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in