Decoupled oxidation process enabled by atomically dispersed copper electrodes for in-situ chemical water treatment
Published in Chemistry and Earth & Environment
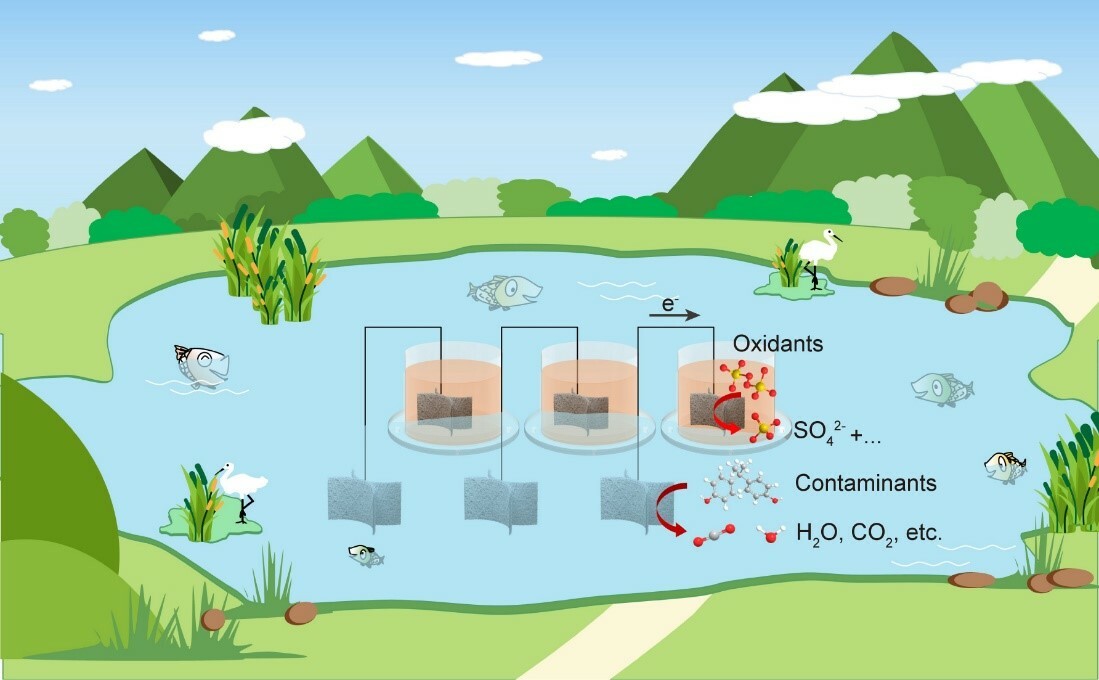
Chemical water treatment with oxidant-involved advanced oxidation processes (AOPs) has shown promising potential for efficiently mineralization of various recalcitrant organic contaminants. However, the introduction of strong oxidants and reactive oxygen species to water may lead to indiscriminate damage to aquatic organisms and ecosystems, as well as potential hazardous by-products. Achieving hazard-free, in-situ water treatment remains a major challenge, especially for surface streams with complex aquatic ecosystems.
Therefore, we have proposed a decoupled oxidation process (DOP) to achieve environmentally friendly chemical water treatment. This approach relies on a high-efficiency catalyst, namely a copper-based single-atom catalyst (Cu-N-C), which creates a substantial potential difference between the oxidative and reductive half-reactions within an AOP. This enables the oxidation of organic pollutants in contaminated water while relegating the reduction of oxidants to an isolated floating chamber, thus preventing direct contact between the aquatic environment and oxidative additives and eliminating potential environmental hazards associated with AOPs .
The setup and performance evaluation of the DOP in the double-chamber galvanic setup demonstrated efficient removal of organic contaminants, with the potential for large-scale water treatment. In our design, organic contaminated water (bisphenol A, BPA, for example) was set as the anodic end while peroxysulphate (PDS) was used as the oxidant for the DOP. The employment of our fabricated Cu-N-C@GF electrode can generate a positive potential difference (ΔE) between the two ends, thus driving the transferring of electrons from organic compounds to PDS, forcing the oxidation reaction to occur. We assessed the DOP's applicability to various organic contaminants (e.g. phenol, p-chlorophenol, and 2,4-dichlorophenol and p-p-nitrophenol). We attributed the specified reaction selectivity to the potential differences between molecules and electrodes of the reaction system. We further ascertained the broad applicability of our approach by fabricating other electrodes (CuO@GF and CNTs@GF) and employed them for the similar reaction. We observed a notable BPA removal capacity in both cases. This experiment underscores the versatility and generality of our DOP strategy.
We investigated the potential application of the DOP by fabricated a floatable water treatment device, and tested its ability to respond to changes in the concentration of organic contaminants and its negligible impact on aquatic ecosystems. Since the reaction only occurs at the co-existence of PDS and BPA, the floatable device possesses a self-responsive characteristic that allows it to respond to changes in the concentration of organic contaminants.
In conclusion, the decoupled oxidation process presents an eco-friendly and efficient method for in-situ water treatment, particularly in remote or isolated regions where centralized water treatment is not feasible. This approach has significant potential for addressing water contamination events and provides a novel, effective, and environmentally friendly in-situ water treatment option.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in