Deubiquitylase USP52 Promotes Bladder Cancer Progression by Modulating Ferroptosis through Stabilizing SLC7A11/xCT
Published in Cancer and Biomedical Research
Bladder cancer (BLCA) ranks as the 10th most common cancer, with an estimated 573 thousand new cases diagnosed and 212 thousand cancer deaths occurring worldwide in 20201. More research efforts are required to explore the potential molecular underpinnings of BLCA tumorigenesis and progression and to further identify diagnostic and prognostic biomarkers to guide the clinical management of BLCA.
Our group developed a tool named EpiTrace tracking single cell evolution via chromatin accessibility2, which revealed the evolutionary trajectories of bladder cancer in all stages3, 4, indicating an intratumor heterogeneity of ferroptosis. Based on these previous results, the current study revealed that USP52 controlled the sensitivity of tumor cells to ferroptosis by stabilizing the xCT protein in a deubiquitinase-dependent manner, which ultimately promoted BLCA progression. We elucidated the role of USP52-xCT axis in BLCA and highlight the therapeutic potential of targeting USP52 and ferroptosis inducers in BLCA.
Ferroptosis, a newly defined iron-dependent regulated cell death (RCD) triggered by excessive lethal build-up of lipid peroxidation on cellular membranes5-7. Solute carrier family 7 membrane 11 (SLC7A11, also known as xCT), a 12-pass transmembrane protein, is the catalytic subunit of the cystine/glutamate transporter system xc- and functions to import cystine into cells via a 1:1 counter transport of glutamate8. A furry amount of evidence demonstrates that the induction of ferroptosis may play a significant role, either alone or in combination, in tumor suppression9, 10.Our study revealed an increase in xCT protein in BLCA. By reanalyzing our scRNA sequencing data, we firstly revealed that BLCA patients with a high ferroptosis state exhibited a lower T stage at single cell level, which suggested that ferroptosis might exert a suppressive effect on BLCA progression in clinical. Notably, ferroptosis inducer IKE (Imidazole Ketone Erastin) treatment exhibited antitumor activity in BLCA xenograft model without any side effects, which further verified a tumor suppressor role of ferroptosis in BLCA in vivo.
Identification and in-depth understanding of specific vulnerabilities in BLCA cells that make them sensitive to ferroptosis provides opportunity for more precise medical treatment of BLCA. Thereout, by screening out an unbiased DUBs RNAi library, USP52/PAN2 was identified as a novel DUB involved in ferroptosis sensitivity and xCT protein regulation. Here, we uncovered an evident increase in the expression of USP52 at transcriptional and translational levels in BLCA tissues compared to that in paracancerous tissues. Besides, patients with high USP52 protein expression had worse overall survival outcomes. Functionally, USP52 depletion impedes glutathione (GSH) synthesis by promoting xCT protein degradation, increasing lipid peroxidation and ferroptosis susceptibility, thus suppressing BLCA progression. Besides, the antitumor activity of USP52 depletion alone was significantly enhanced by ferroptosis induction in vivo, which suggested a sound therapeutic strategy of BLCA patients with high USP52 level.
Mechanistically, USP52 physically associated with xCT through its WD40 domain, leading to xCT K48-conjugated deubiquitination at K4 and K12, which in turn promoted xCT protein stability. Notably, no reports have identified any E3 ligase-mediated ubiquitination of the xCT protein at K4 or K12, leaving an open question regarding the identification of another antagonistic regulator responsible for ferroptosis sensitivity and ubiquitination modification of xCT at K4 or K12 in BLCA in future studies. To ensure proper function of xCT-mediated cystine transport and subsequent redox balance, the expression and activity of xCT are strictly regulated by several types of posttranslational modifications (PTMs)11-13. Palmitoylation, as another one of the most common PTMs, might promote downstream protein degradation by affecting protein ubiquitination, and xCT can be palmitoylated by ZDHHC8 at C32711. Based on this, it will be interesting to investigate that whether or under what conditions USP52-modified xCT deubiquitination is influenced by ZDHHC8-mediated palmitoylation.
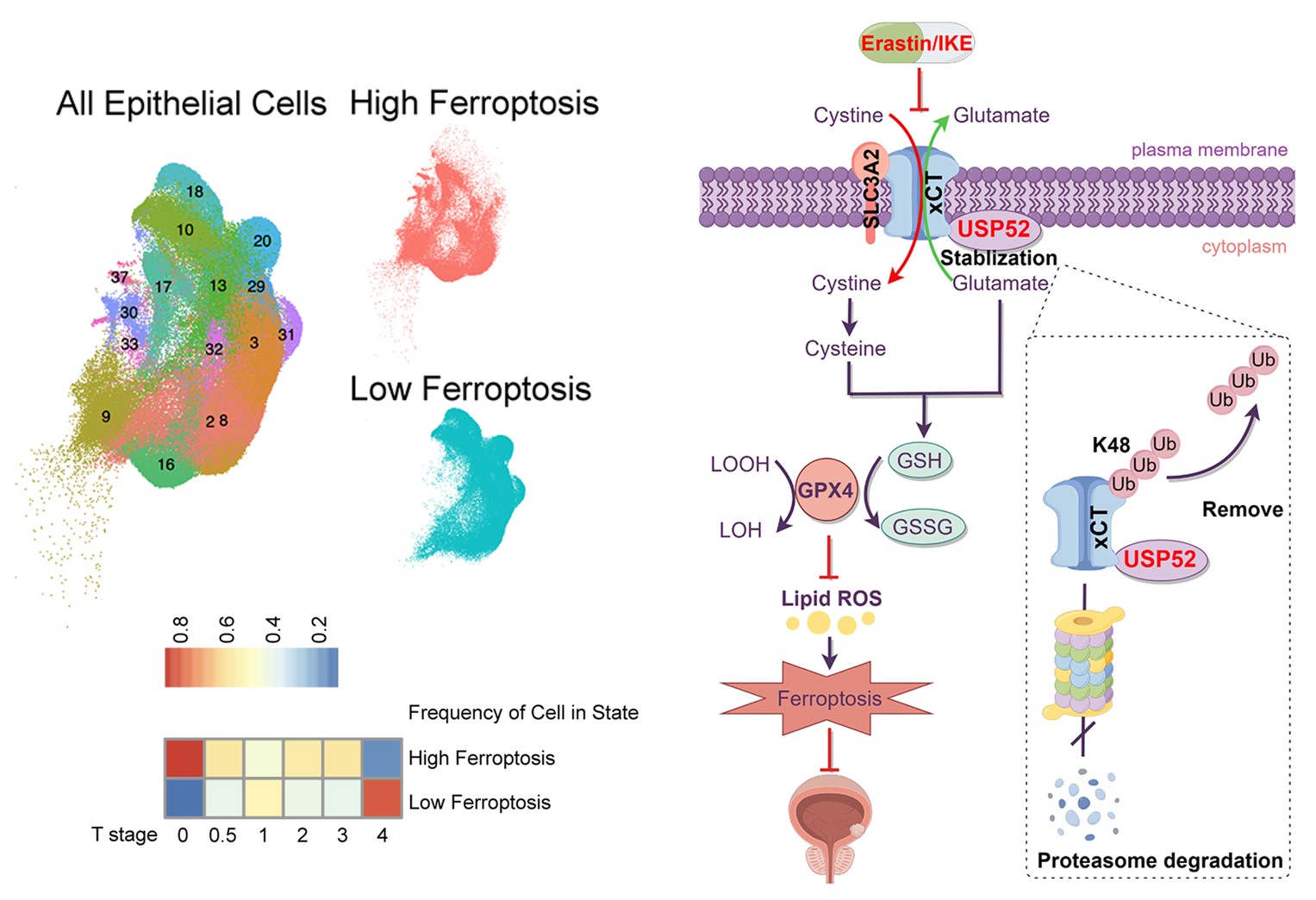
Overall, with respect to cultured cell lines, animal tumor-bearing models and clinical human samples, we revealed that USP52 controlled the sensitivity of tumor cells to ferroptosis by stabilizing the xCT protein in a deubiquitinase-dependent manner, which consequently facilitated BLCA progression. Genetic blockade of USP52 and/or IKE might be a potential strategy for developing BLCA-targeting therapies.
Dr. Kaiyu Qian and Dr. Xinghuan Wang are the corresponding authors of this paper, with MD candidate Jianmin Liu, Dr. Yongwen Luo, MD candidate Simin Chen and Dr. Gang Wang serving as co-first authors.
References
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71, 209-249 (2021).
2. Xiao, Y. et al. Tracking single-cell evolution using clock-like chromatin accessibility loci. Nat Biotechnol (2024). doi: 10.1038/s41587-024-02241-z. Epub ahead of print.
3. Xiao, Y. et al. Integrative Single Cell Atlas Revealed Intratumoral Heterogeneity Generation from an Adaptive Epigenetic Cell State in Human Bladder Urothelial Carcinoma. Adv Sci (Weinh), e2308438 (2024).
4. Wang, Y. et al. DNA polymerase POLD1 promotes proliferation and metastasis of bladder cancer by stabilizing MYC. Nat Commun 14, 2421 (2023).
5. Dixon, S.J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060-1072 (2012).
6. Xie, Y. et al. Ferroptosis: process and function. Cell Death Differ 23, 369-379 (2016).
7. Stockwell, B.R. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273-285 (2017).
8. Koppula, P., Zhang, Y., Zhuang, L. & Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 38, 12 (2018).
9. Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18, 280-296 (2021).
10. Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer 22, 381-396 (2022).
11. Wang, Z. et al. AMPKα1-mediated ZDHHC8 phosphorylation promotes the palmitoylation of SLC7A11 to facilitate ferroptosis resistance in glioblastoma. Cancer Lett 584, 216619 (2024).
12. Gu, Y. et al. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Mol Cell 67, 128-138.e127 (2017).
13. Tang, J. et al. Targeting USP8 Inhibits O-GlcNAcylation of SLC7A11 to Promote Ferroptosis of Hepatocellular Carcinoma via Stabilization of OGT. Adv Sci (Weinh) 10, e2302953 (2023).
Follow the Topic
Your space to connect: The Cancer in understudied populations Hub
A new Communities’ space to connect, collaborate, and explore research on Cancers, Race and Ethnicity Studies and Mortality and Longevity!
Continue reading announcement
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in