Development and evaluation of a novel lateral flow immunoassay for rapid diagnosis of brucellosis across different animal species
Published in Biomedical Research, General & Internal Medicine, and Zoology & Veterinary Science
More than a century has passed since David Bruce discovered the agent that causes Malta fever, and brucellosis is still one of the major zoonotic illnesses that cause significant cattle reproductive failure and financial losses in the dairy industry, with global distribution. A classic example of a zoonotic disease that is common worldwide and has been identified by the World Health Organization (WHO) as the most neglected zoonotic disease, brucellosis, which is caused by several species of the genus Brucella. It is still a major epidemic in low- and middle-income countries. It is more frequently associated with the employment and intake of animal-based meals. Consequently, the largest risk groups include veterinary professionals, animal farmers, and slaughterhouse workers, who typically contract infections through mucous or abraded skin. People who breathe in contaminated dust or airborne droplets, such as those who work in the fur processing industry, are susceptible to respiratory tract diseases. Most often, contaminated food or water causes gastrointestinal tract infections in people who have never been around farm animals or animal products.
In terms of endemic regions and time, the burden of brucellosis infections varies. Despite this dynamic, the one constant in the chain of transmission is that human cases of brucellosis are invariably associated with certain animal reservoirs. The course and outcome of brucellosis are greatly impacted by early detection and laboratory confirmation of the diagnosis because the disease typically manifests in a variety of nonspecific ways. Since brucellosis cannot be identified without solid microbiological, molecular, and epidemiological data, a coordinated approach to diagnosis and control is always necessary. Controlling brucellosis in animals and, by extension, humans, requires an accurate diagnosis. One of the main challenges to eliminating brucellosis is accurate diagnosis. Because of the significant risk of laboratory-acquired infections, routine identification and discrimination of brucellosis-suspected specimens based on culture isolation and phenotypic characterization necessitates biosafety level 3 (BSL-3) techniques.
Brucellosis management presents several diagnostic difficulties. First, the infection may spread and become sustainable if it spreads to a larger variety of animal hosts through culture-based spillover. The management of this persistent and widespread infection necessitates the creation of diagnostic tests that can get past the drawbacks of current methods, such as the requirement for sophisticated equipment and skilled technicians, which are unavailable in many diagnostic situations, and low sensitivity and/or specificity, which are additional shortcomings that could produce unreliable results. Diagnostic methods for brucellosis still commonly include SAT, RBPT, CFT, and ELISA. For more accurate identification and to prevent false positives, it is often advised to utilize the RBPT in conjunction with other common serological tests. Although CFT was created to detect IgG, its cross-reactivity with calves who have received the B. abortus S19 vaccine makes it primarily a confirming test. Because ELISA has better sensitivity and specificity than SAT, it is frequently used to identify partial antibodies and diagnose chronic cases of brucellosis.
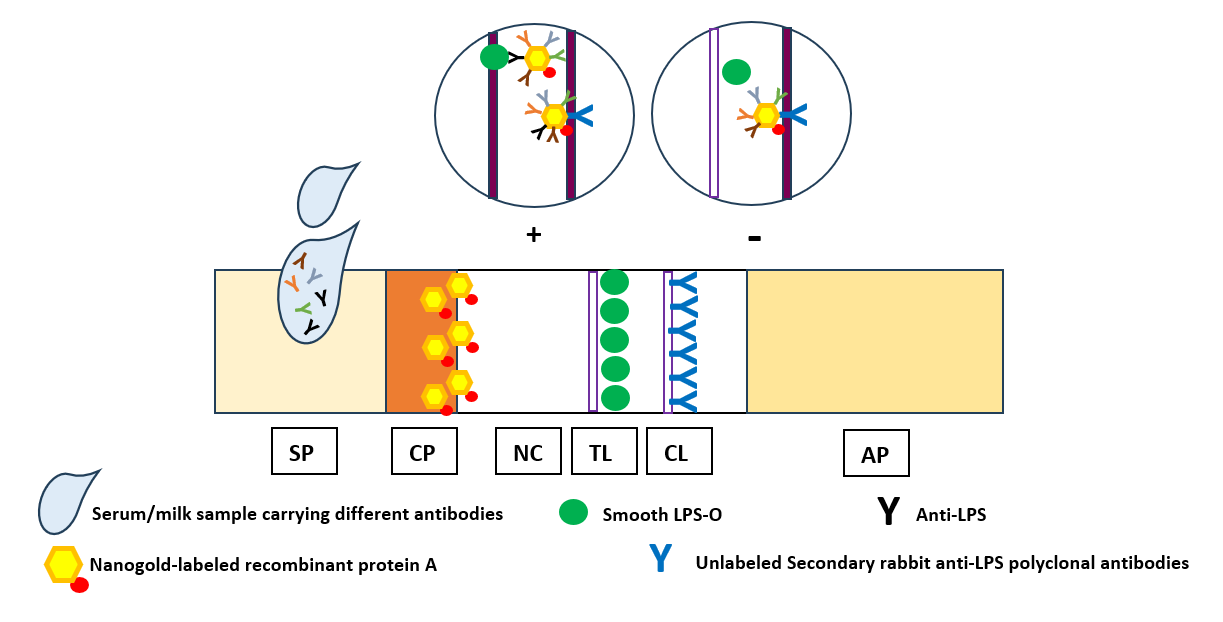
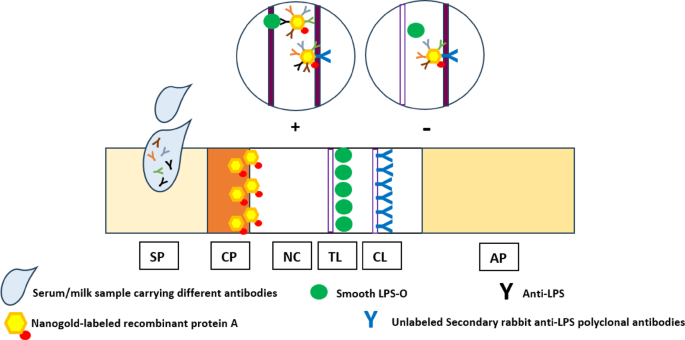
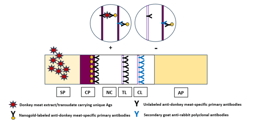
To enhance diagnostic capabilities, a variety of PCR-based assays have been created for the identification of Brucella. When taken as a whole, the test repertoire covers several diagnostic process facets. Differential PCR-based tests are typically more complicated and, as a result, more challenging to execute. Numerous methods, such as locus-specific multiplexing (AMOS-PCR based on IS711), PCR-RFLP (the omp2 locus), arbitrary-primed PCR, and ERIC-PCR, have been investigated to distinguish between Brucella species and strains. A verification test is frequently necessary, and none of the diagnostic methods that have been developed and are currently accessible satisfy the conventional criteria for a convincing diagnosis. Additionally, none of the assays are advised to be used alone in endemic areas. The LFIA, which is used to identify immunoglobulin IgM and IgG-specific antibodies (Ab) against LPS, is one of the quick and accurate diagnostic tests.
This study’s objective is to:
-
(1)
Develop and evaluate a novel sero-diagnostic with the affordability, sensitivity, specificity, user-friendliness, rapid and reliable performance, equipment-free operation, and deliverability to those in need (ASSURED) criteria for brucellosis diagnosis in comparison to PCR and a wide range of conventional serological assays (RBPT, SAT, MRT, and I-ELISA as screening tests and C-ELISA and CFT as confirmatory tests).
-
(2)
Illustrate how the purity of LPS and non-specific immunoglobulins significantly impacts the diagnostic specificity and sensitivity of sero-diagnostics and offer a promising way to mitigate this impact using the newly designed assay’s suggested format.
-
(3)
Improve the common sero-diagnostic LFIA based on the conjugates of immunoglobulin-binding proteins with traditional nano-dispersed labels that ensure an increase in labeled immune complexes in the test line, which, by its role, reflects on the newly developed LFIA sensitivity and range of detection to a different and wide range of Brucella spp. in different animal species by the same developed diagnostic assay.
Brucellosis is an endemic bacterial zoonosis worldwide and is among the most prioritized zoonotic diseases. It is commonly known that brucellosis has an impact on dairy production and public health in both industrialized and developing nations. The majority of human and livestock cases are not properly detected by the current surveillance systems, which leads to a major underestimation of the disease burden. Additionally, the use of nonspecific antibiotics to treat infected cases may worsen the situation later on, as evidenced by antimicrobial resistance. Due to the clinical, zoonotic, and economic huge effects of brucellosis, several phenotypic, genotypic, and serological diagnostic techniques were developed, and different integral diagnostic protocols were adopted in both national and international control strategies. Despite the deep-rooted process of development of brucellosis diagnostics, till now, none of the developed techniques can be considered the sole diagnostic tool for brucellosis that covers all the proper diagnostic criteria at once. Therefore, working in the field of brucellosis diagnosis development and improvement was and remains a very questionable field of science, full of gaps that need to be properly filled with a reasonable contribution. The diagnostic approaches for brucellosis can be categorized into three main categories: culture-based phenotypic identification, molecular-based genotypic techniques, and a wide range of conventional serological assays.
Bacterial culture is a gold standard test for confirmation of Brucella; however, in addition to a wide range of sensitivity, from 10 to 90%, this method is recognized as a high-risk method for laboratory personnel. Molecular biotechnology techniques such as real-time or Amplification of Multiple Loci by “Abortus, Melitensis, Ovis, and Suis” polymerase chain reaction (AMOS-PCR) are designed to quantitatively detect small amounts of bacterial DNA or differentiate Brucella strains, which are reliable if precise and expensive equipment is available. In recorded that the limit of detection for B. abortus in most matrices was in the range of 103–104 CFU/g for cultivation and 104–105 CFU/g for direct real-time PCR. Also, the target gene (IS711) confirms the presence of Brucella on the genus level: Southern blot analysis determined the distribution of IS711 in the Brucella species, showing the presence of at least one potentially unique copy of the IS711 gene in every species except B. canis. IR1 and IR2, primer sequences for the Brucella genus, were used in the current study on coupled 160 milk and serum samples to correlate the PCR detection of Brucella from serum and milk samples with MRT and the newly developed LFIA. The obtained results represent an observable agreement and lower dissociation between the two raters (PCR and MRT) which also can be accounted for by the newly developed LFIA due to the low specificity, sensitivity, and agreement of the MRT with the C-ELISA in comparison with the newly developed LFIA.
Intending to develop and improve serology-based diagnostic strategies, research has focused on understanding the pathogenic determinants that allow Brucella to establish successful chronic infections and evade immune eradication. Brucellae carry a cell surface lipopolysaccharide whose immunodominant section induces an antibody response that may be difficult to distinguish from that resulting from a true infection, this complicates serodiagnosis because the tests currently used detect antibodies to the LPS. LPS is considered the main bacterial antigen responsible for inducing the expression of pro-inflammatory molecules and the main target for the humoral antibacterial response during the infection of the host. The whole cell or most often the LPS as a major Brucella virulence factor, is commonly used for serological assays development; however, cross-reactivity of antibodies against the Brucella LPS chain with other Gram-negative bacteria such as E. coliO157, V. cholera, F. tularensis, and Y. enterocolitica results in low specificity of these techniques.
RBPT, SAT, I-ELISA, and MRT as screening tests and C-ELISA and CFT as confirmatory tests are the most popular methods that rely on using either whole bacterial antigen or LPS for the detection of anti-Brucella antibodies. Also, despite the wide range of testing limitations of MRT, the World Organization of Animal Health (WOAH) recommended MRT for monitoring the occurrence of bovine brucellosis at the herd level, which consists of a screening of specific antibodies against B. abortus in bulk milk tanks. The newly developed LFIA showed very competitive comparative results with the MRT by means of sensitivity, specificity, and accuracy, with a major advantage, which is the different sample types testing applicability in favor of the newly developed LFIA. Also, it has shown better positive and negative predictive values than all compared diagnostic techniques in the current study, suggesting that the test is simple, cost-effective, and rapid, and provides accurate detection of antibodies to brucellae in the examined samples. This rapid test can therefore be practically implemented in serological screening for brucellosis, although evaluation on a larger scale with various animal sera, blood, and milk samples is still necessary.
Although the currently developed LFIA is not the first lateral flow immunochromatography-based technique to be developed for the diagnosis of brucellosis, competitive advantages-based modifications have been incorporated in the newly introduced LFIA that have efficiently reflected on its diagnostic capabilities in comparison with seven different diagnostic assays. In the current study, modifications have been employed for the extraction and purification of S-LPS-O from B. abortus, which would allow early elimination of contaminating components to enhance purity results in highly pure S-LPS-O free of protein and nucleic acids. Using colloidal nanogold, which is still the most dominant colorimetric label. As well as using rabbit anti-LPS antibodies on the control line, enhance the signal (color intensity) of the control line by additionally binding to the idiotypic determinants (idiotypes) of the anti-idiotypic antibodies of the test serum, which is another novelty point of the newly developed LFIA. Recombinant protein A-conjugated nanogold was successfully incorporated, for the first time, as the bioconjugate giving the newly developed LFIA a very important diagnostic advantage which is that the detection ability became not dependent on specific Brucella sp., immunoglobulin class, sample type, and/or animal species as protein A has the ability to interact with different immunoglobulins classes of most mammalian species in a wide range of samples. This permits indirect antibody assays with sera for which specific immunoglobulin is not readily available and permits the detection of multiple antigens in indirect immunoassays utilizing antisera from several different species.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in