Diagnostic Dilemma: Tackling Challenges of CNS-Originating Extracellular Vesicle Biomarkers in Distinguishing Parkinson's Disease and Multiple System Atrophy
Published in Neuroscience

The human body is made up of a myriad of different types of cells, each with its own specific function. Some cells, such as neurons, are responsible for transmitting electrical impulses that allow us to move and think; others, such as immune cells, help protect us from disease and infection. Despite their diverse functions, all of these cells must work together in harmony for our body to function properly.
So how do these cells communicate with each other? One way is through direct contacts, such as when immune cells recognize and destroy infected cells. Another way is through the release of chemical messengers called cytokines, which can signal neighboring cells to take action. But perhaps the most fascinating form of cell-to-cell communication is through the use of extracellular vesicles (EVs).
EVs are small, bubble-like structures (see Figure 1) that are released by cells and can travel throughout the body. They contain a variety of molecules, such as proteins, lipids, and nucleic acids, which can be transferred to other cells. What's particularly fascinating is that EVs are lipophilic and can move across the blood-brain barrier, a protective membrane that prevents most molecules from entering or leaving the brain, to the peripheral circulation – providing a unique window into the biochemistry of the brain. This allows cells to communicate with one another in a highly targeted and specific way. For instance, neurons can use EVs to transmit signaling molecules to other neurons, enabling complex communication between different parts of the brain and cells in the body.
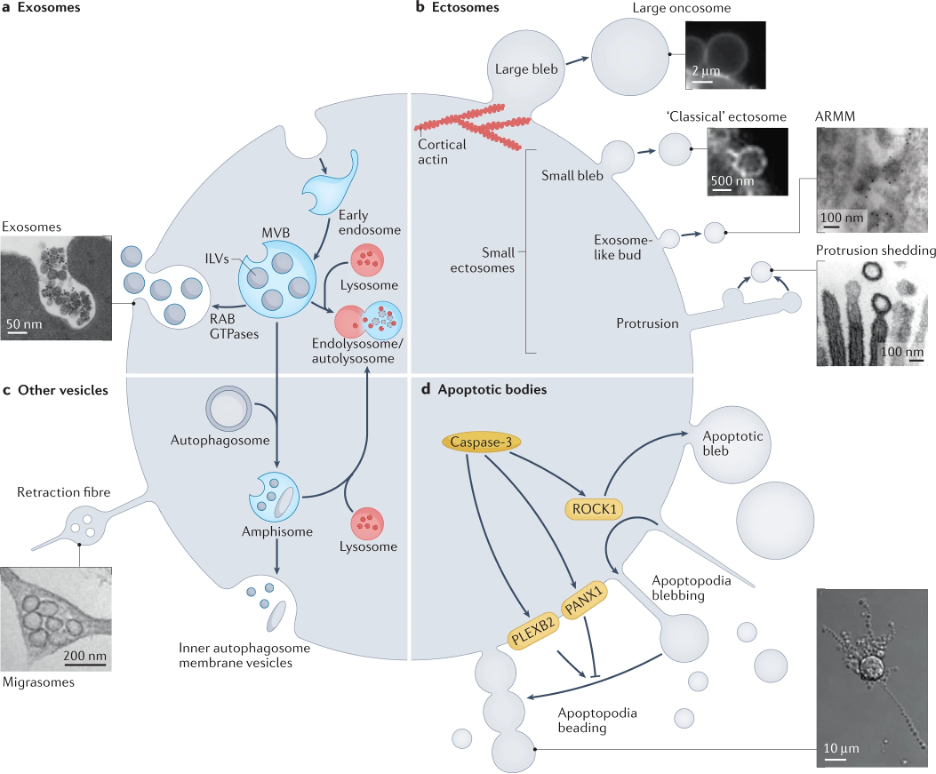
Figure 1. Different types of tiny-bubble-like extracellular vesicles (EVs) and their biogenesis pathways. Adapted from Dixson et al. Nat Rev Mol Cell Biol, 2023; https://doi.org/10.1038/s41580-023-00576-0
Parkinson's disease (PD) and multiple system atrophy (MSA) are both neurodegenerative diseases characterized by the accumulation of toxic α-synuclein (α-syn) protein in the body, leading to the death of cells. However, PD primarily affects neurons, while MSA affects both neurons and a specific type of supporting cell called oligodendrocytes. Despite this difference, the two diseases share similar parkinsonism symptoms and are often misdiagnosed by neurologists with each other and with other diseases (as shown in Figure 2), which can hinder appropriate treatment management and discovery. Unfortunately, the only way to confirm the correct diagnosis is through a pathological exam after the patient has passed away
Therefore, this study aimed to establish a minimally-invasive blood test for accurate differential diagnosis of both diseases, similar to how Type-2 diabetes is diagnosed through a blood test measuring blood sugar levels, by measuring levels of α-syn, phosphorylated α-syn at Ser 129 (pS129-α-syn - a common variant of the protein in these diseases), and tau (a protein affected in PD) in both neuronal and oligodendroglial extracellular vesicle (nEVs and oEVs, respectively). With a reliable blood test for PD and MSA, doctors may be able to diagnose these diseases earlier and provide more effective treatments to improve patient's quality of life. This has the potential to greatly impact patient outcomes, as misdiagnosis can lead to inappropriate treatment and management, while earlier and more accurate diagnosis can allow for earlier intervention and improved quality of life for patients
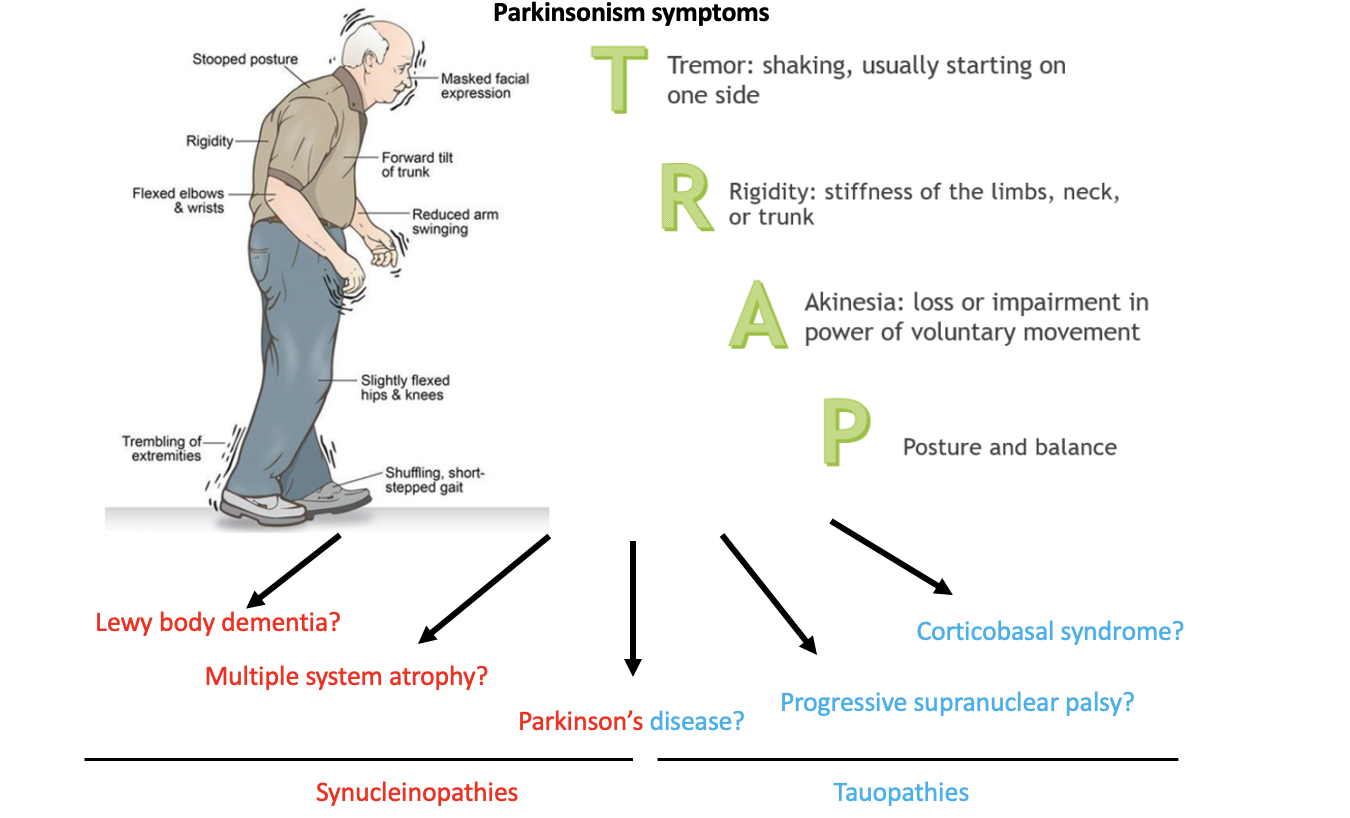
Figure 2. Parkinsonism symptoms affect a heterogenous group of neurodegenerative diseases. These diseases are often misdiagnosed premortem and accurate diagnosis can only be confirmed postmortem. The top image is adapted from https://parkinsonsnebraska.org/understanding-parkinsons-disease/.
Previously, it was found that the ratio of α-syn in oEVs to nEVs was greater than 1 in most patients with MSA, whereas in most patients with PD, it was below 1 (Dutta et al. Acta Neuropatholog, 2021). Here using a subset of these patients, we found that levels of pS129-α-syn were higher in oEVs from patients with MSA compared to those with PD. Furthermore, both nEVs and oEVs tau levels were lower in patients with MSA compared to those with PD. Using an algorithmic model, we were able to differentiate between the two diseases with high accuracy by combining nEVs α-syn, oEVs:nEVs α-syn, oEVs pS129-α-syn, and EV particle concentration, with an accuracy of 93.6% (Figure 3). These findings are promising but are subject to certain limitations.
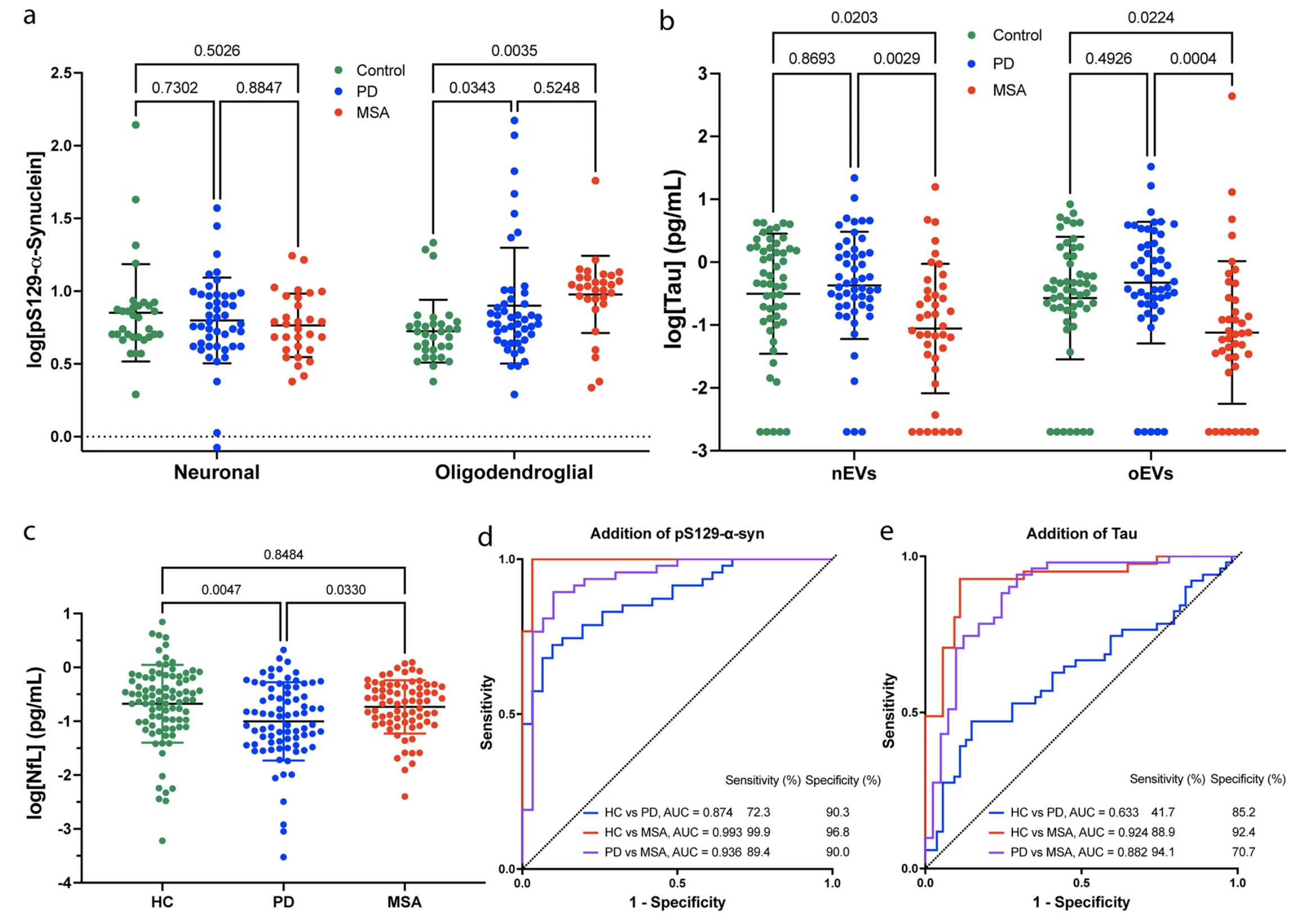
First, it's important to note that some of the measurements of the protein levels contained high overlap, which can lead to errors in interpretation. Additionally, EVs can be affected by a range of preanalytical factors, including the choice of anticoagulant used with plasma, the time of preparation, centrifugation methodology, the nature of transport, number of freeze/thaw cycles, storage conditions, temperature, and the type of serum collection tube. As this study is exploratory in nature, it was not possible to control for these factors. Furthermore, the antibody used for measuring pS129-α-syn (EP1536Y), which is a common variant of α-syn found in patients with PD and MSA, has come under scrutiny by Hilal Lashuel’s group in a recent publication (https://www.nature.com/articles/s41531-022-00388-7) and may not provide accurate measurements of pathological α-syn in EVs. Therefore, further validation of similar antibodies is needed to confirm the findings of this study. It's also worth noting that the results have not been validated by other groups or in independent cohorts.
Lastly, some patients have passed away, and the postmortem diagnosis did not always match the results of the test. This highlights the need for more accurate and reliable diagnostic tools for PD and MSA, especially as misdiagnosis can lead to inappropriate treatment and management. While the results of this study are promising, it's important to approach them with caution and wait for further validation by other research groups while addressing all of the issues mentioned above. Ultimately, this study may represent an important step toward the possibility of the development of a blood test for the accurate diagnosis of these devastating neurodegenerative diseases.
Follow the Topic
-
Translational Neurodegeneration

An open access, peer-reviewed journal that covers research, therapeutics and education for all aspects of neurodegenerative diseases.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in