Dietary amino acids promote glucagon-like hormone release to generate global calcium waves in adipose tissues in Drosophila
Published in Earth & Environment, Ecology & Evolution, and Protocols & Methods
Energy balance in the body is tightly regulated by hormones like insulin and glucagon. While a substantial amount of research has focused on understanding insulin, considerably less is understood regarding glucagon. Glucagon plays a critical role in increasing glucose production in the liver (gluconeogenesis) and breaking down fats (lipolysis) during energy shortages. To mobilize fat stored in adipose tissue, glucagon interacts with receptors on the liver and fat cells. These receptors, which belong to the GPCR family, trigger a rise in calcium levels inside cells. This increase in calcium activity initiates a chain reaction leading to fat breakdown. Despite these findings, many questions remain unanswered about how glucagon is regulated, particularly the spatial-temporal dynamics of glucagon signaling and its underlying mechanisms, as well as its role beyond starvation.
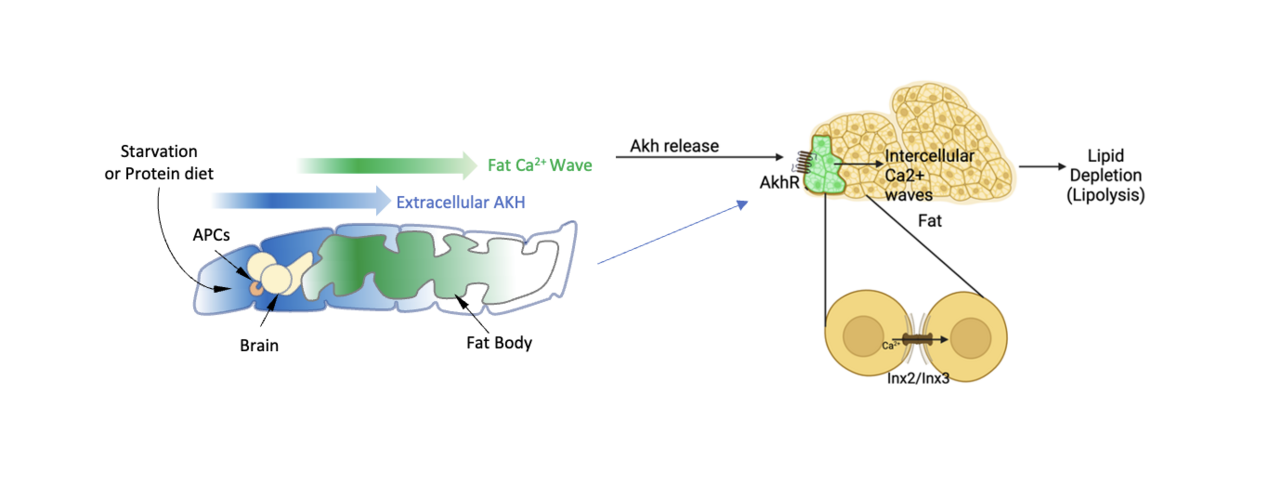
In recent years, the fruit fly (Drosophila) has become an important model for studying hormonal signaling, especially insulin and glucagon. In flies, Adipokinetic Hormone (AKH) serves a comparable function to that of glucagon. Under starvation conditions, AKH is released from specialized neurons called Akh-producing cells (APCs). This hormone signals to fat tissues by binding to its receptor AkhR, which also belongs to the GPCR family. Similar to the mammalian glucagon receptor, when AkhR is activated, calcium levels in fat tissue cells increase, prompting the breakdown of stored fats.
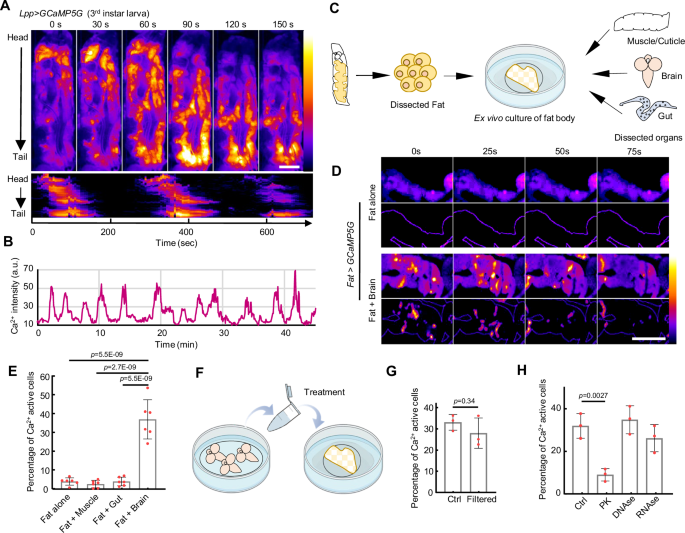
One fascinating mechanism that coordinates this response across tissue is the propagation of intercellular calcium waves (ICWs). These waves allow cells to respond collectively to hormonal signals. Although ICWs have been observed in fly fat tissue, the exact trigger in vivo was previously unknown. This project started with an unexpected discovery that global ICWs travel in the fat cells from the fly larval head to tail, which has not been reported before. Using live imaging techniques, we tracked ICWs in real-time within fly fat tissue and identified AKH as the key driver of these calcium dynamics. Through genetic experiments, computational modeling, and tissue studies outside the body (ex vivo), we showed that both AKH and its receptor AkhR are essential for ICWs in fat tissue. Surprisingly, we found that these waves are synchronized by a novel mechanism that does not rely on gap junctions (connections between cells) and still effectively promotes fat breakdown.
Next, we investigated what causes APCs to release AKH. While starvation is a well-known trigger, we explored whether dietary factors might also play a role. In mammals, high-protein diets have been linked to glucagon release. However, it is not clear what specific dietary amino acids may trigger this response. We discovered that specific amino acids—including methionine and threonine—can stimulate APCs to release AKH in flies. This AKH release, in turn, drives calcium waves in fat tissue, resulting in fat breakdown. These findings reveal that dietary amino acids are key regulators of AKH release, providing a direct link between diet and fat metabolism.
Our study demonstrates a new mechanism by which dietary amino acids can influence fat metabolism through AKH signaling, which paves the way for future studies on dietary regulation of metabolism and hormone signaling. By uncovering the role of AKH in driving calcium waves and lipolysis via a novel, gap-junction-independent process, our work also demonstrates new ways to study how diet affects metabolism in a real-time manner. This, in turn, may facilitate innovations to tackling obesity and related diseases.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in