Double Boron–Oxygen-Fused Polycyclic Aromatic Hydrocarbons: Skeletal Editing and Applications as Organic Optoelectronic Materials
Published in Chemistry
Introduction the Development of Double Boron–Oxygen-Fused Polycyclic Aromatic Hydrocarbons (dBO-PAHs)
Polycyclic aromatic hydrocarbons (PAHs) have been widely used in organic electronics and organic photovoltaics owing to their unique optoelectronic properties. Recently, boron-embedded PAHs (B-PAHs) and boron/nitrogen-embedded PAHs (BN-PAHs) are found to exhibit outstanding photophysical properties and attracte enormous research interest. However, scarce knowledge is available on PAHs containing B–O unit (BO-PAHs), and in particular on their optoelectronic properties, due to their inefficient synthetic methods.
Their molecular configurations, as well as photophysical and excited-state properties, are systematically studied by X-ray crystallography, theoretical calculations, optical spectroscopy, and device physics. The doubly embedded B–O units can efficiently regulate the photophysical and excited-state properties, enabling the dBO-PAHs to act as robust emitters, ultralong afterglow materials, or n-type host materials for high-brightness and high-efficiency deep-blue OLEDs.
One-Pot Synthesis of dBO-PAHs and Facile Skeletal Editing
In this work, we design several series of novel double boron–oxygen-fused PAHs (dBO-PAHs), and a facile synthesis of dBO-PAHs via an efficient one-pot strategy with high regioselectivity and efficient skeletal editing is developed (Fig. 1). Through this method, six types of dBO-PAHs (20 compounds, 9 single crystal X-ray structures) have been facilely synthesized, and most of them can be synthesized in gram-scale amounts (up to 16.3 g for BO1c) (Fig. 2); they are extremely stable, insensitive to air and water, and also possess highly thermal stabilities.
Single-Component, Dual-Emissive Materials, and Ultralong Low-Temperature Phosphorescence.
Single-component, dual-emissive materials are highly desirable because of their potential applications in bio-imaging, ratiometric/optical sensing, and oxygen detection. Moreover, ultralong organic phosphorescence (UOP) materials have been extensively developed via host-guest doping, crystallization, polymerization, etc.; however, single-component UOP materials with long second-level phosphorescence lifetimes (τp) are rare.
Then, their photophysical properties are investigated. The dBO-PAHs exhibit single-component, dual-emissions at 77 K in 2-MeTHF with nanosecond fluorescence (τf = 1.2–12.6 ns) and second-level UOP (τp = 0.3–5.0 s); importantly, the long τp endows the dBO-PAHs to exhibit low-temperature ultralong afterglow of up to 20 s after UV light excitation (Fig. 3). This enables the promising applications in the areas of surface icing indications, optical thermometry, biological labeling, and virus preservation in extreme temperature conditions. Moreover, the dBO-PAHs exhibit strong fluorescence emissions at room temperature in dichloromethane with high-color-purities [full-width at half-maximum (FWHM) = 25–52 nm], excellent quantum efficiencies (ΦPL up to 95%), and large radiative rates (kr up to 6.8×108 s-1), making them act as robust blue to ultraviolet (UV) emitters.
Application as n-Type Host Materials for Deep-Blue OLEDs
Host material is critical for the device performances of deep-blue organic light-emitting diodes (OLEDs); however, very few host materials can be selected because of the high T1 energy levels of the deep-blue emitters, which seriouly impedes the development of high-performance deep-blue OLEDs. Therefore, developing novel and robust materials, which can solve the above problems, is an extremely urgent yet challenging task.
BO1b-based deep-blue OLED exhibited higher maximum external quantum efficiency (EQE) (22.8%) than those of traditional hosts 26mCPy, mCP and mCBP-based OLEDs (21.0%, 18.5% and 16.3%, respectively). Importantly, compared to single host, BO1b:mCBP co-hosts-based deep-blue OLEDs exhibited dramatically brightness and efficiency enhancements with significantly reduced efficiency roll-offs (Fig. 4). Device 10 using PtON1 as emitter achieved a maximum brightness (Lmax) of 27219 cd/m2 with a peak EQE of 27.8%, which represented the record-high Lmax among Pt(II)-based deep-blue OLEDs with CIEy < 0.20. Device 9 using PtON-TBBI as emitter demonstrated a 4.6-fold Lmax enhancement with a high color-purity (CIEy = 0.104), and also achieved the record-high EQE (28.0%) among Pt(II)-based deep-blue OLEDs. Device 9 represents the best comprehensive performance of deep-blue OLEDs (Fig. 5). These results suggest that dBO-PAHs can act as robust n-type host materials for deep-blue OLEDs.
Summary and Outlook
In this study, we present an efficient one-pot strategy for the synthesis of dBO-PAHs with high regioselectivity and efficient skeletal editing. The potential applications of the dBO-PAH as ultralong organic phosphorescence and n-type host materials for deep-blue OLEDs are investigated. We believe this study provides important insights into the unique optoelectronic properties of the dBO-PAHs.
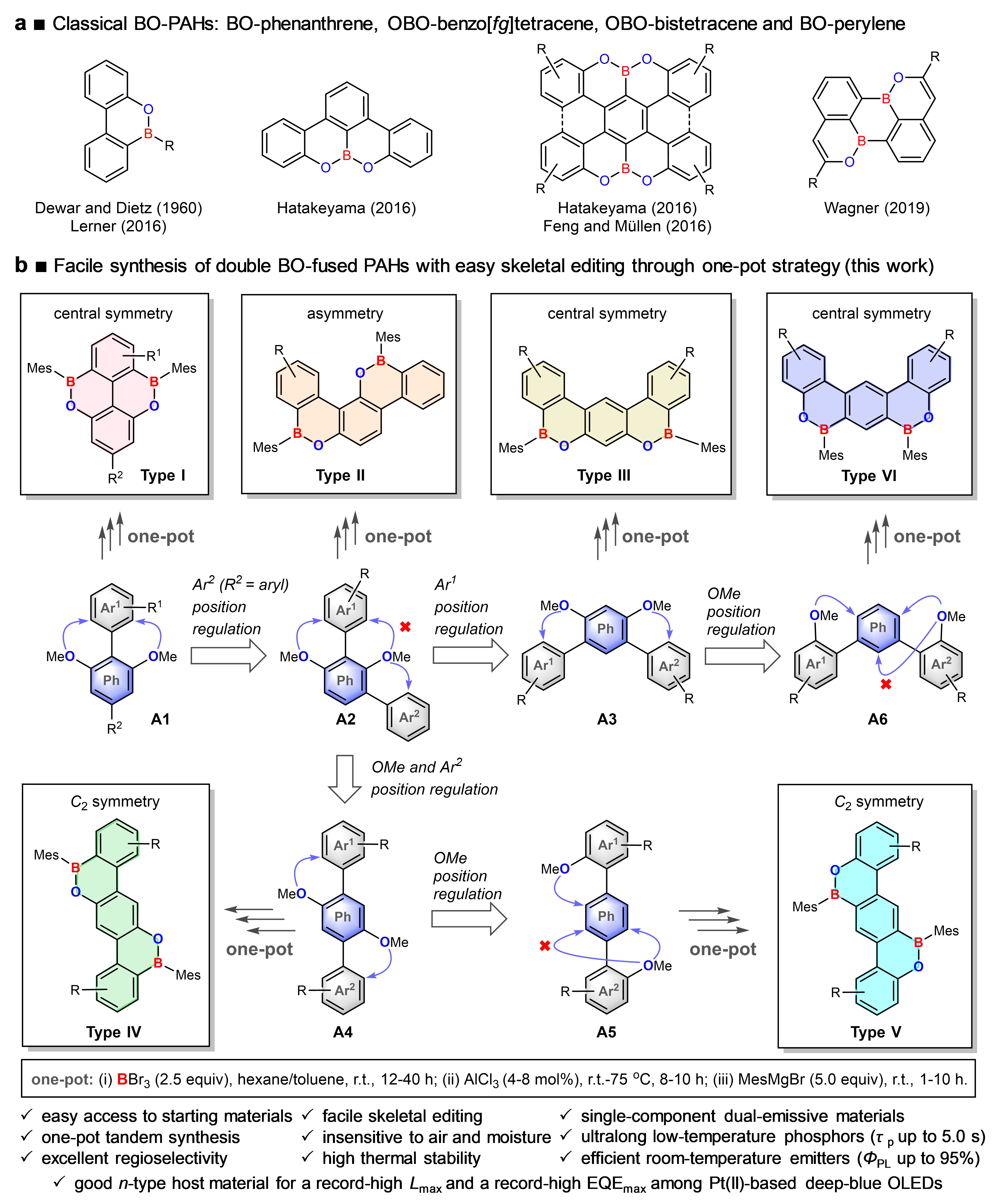
Fig. 1 Schematic illustration of the current work. a Previous reported BO-PAHs. b Design concept of versatile skeletal editing and facile synthesis of dBO-PAHs through one-pot strategy developed in this work. The versatile skeletal editing can be easily realized by Ar1, Ar2 or OMe position regulation; the arrows indicate the position of electrophilic borylation directed by the OMe group. Ph, phenyl group; Mes, 2,4,6-trimethylphenyl group; Ar1, Ar2, aryl group; R, R1, R2, substituent group.
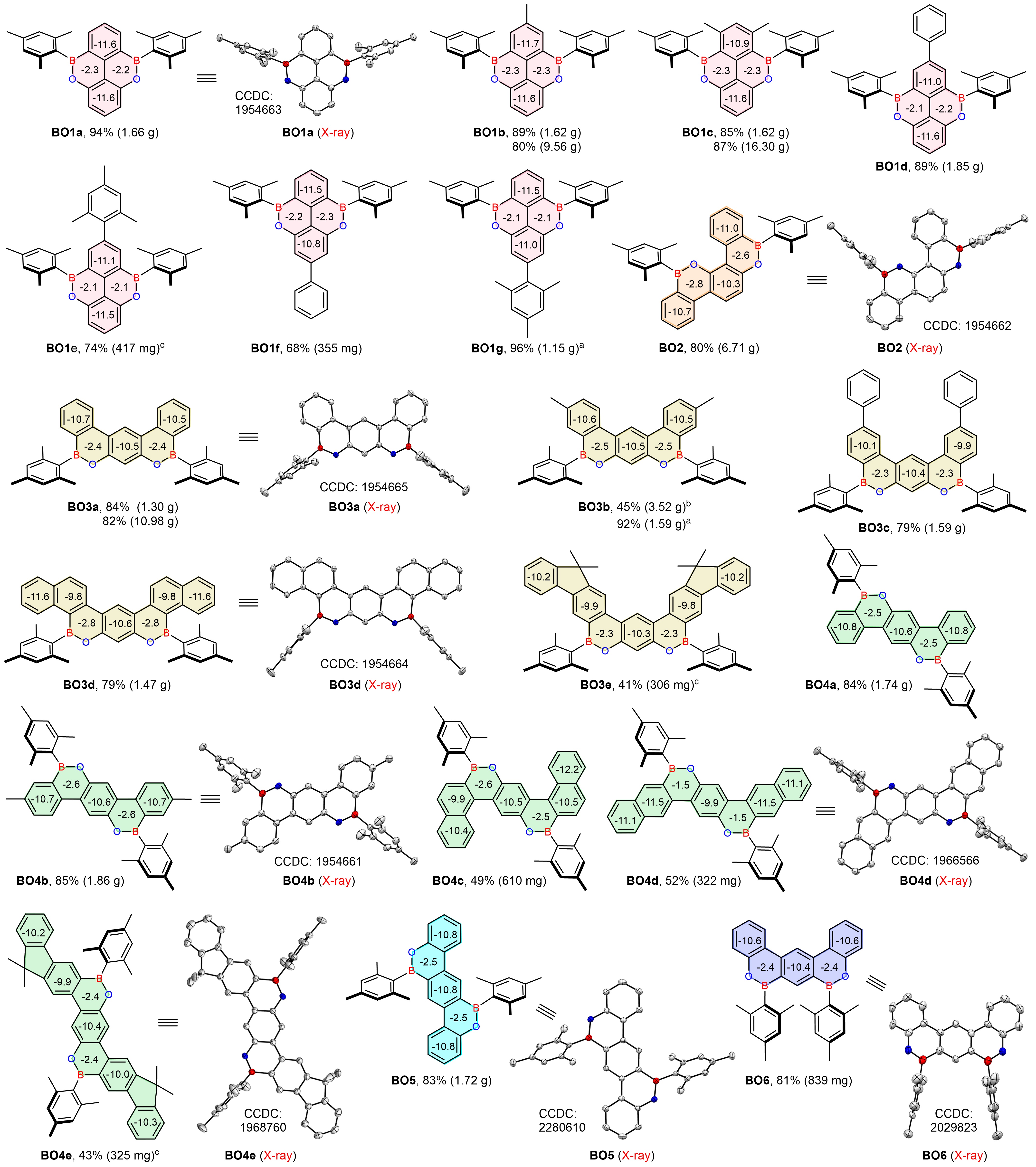
Fig. 2 Skeletal editing and theoretical calculation. The dBO-PAHs synthesized via one-pot protocol with 4 or 8 mol% AlCl3 was used. The single crystal X-ray structures are shown with thermal ellipsoids at 50% probability, and hydrogen atoms are omitted for clarity. NICS(1) values calculated with a B3LYP/6-31G(d) basis set in the gas phase are shown. The obtained product weight was provided in parentheses. a20 mol% AlCl3 was used. bA by-product 6-mesityl-8-methyl-2-(p-tolyl)-6H-dibenzo[c,e][1,2]oxaborinin-3-ol (BO3b-OH, CCDC: 2290997) was also isolated in 41% yield. c2.0 equiv AlCl3 was used.
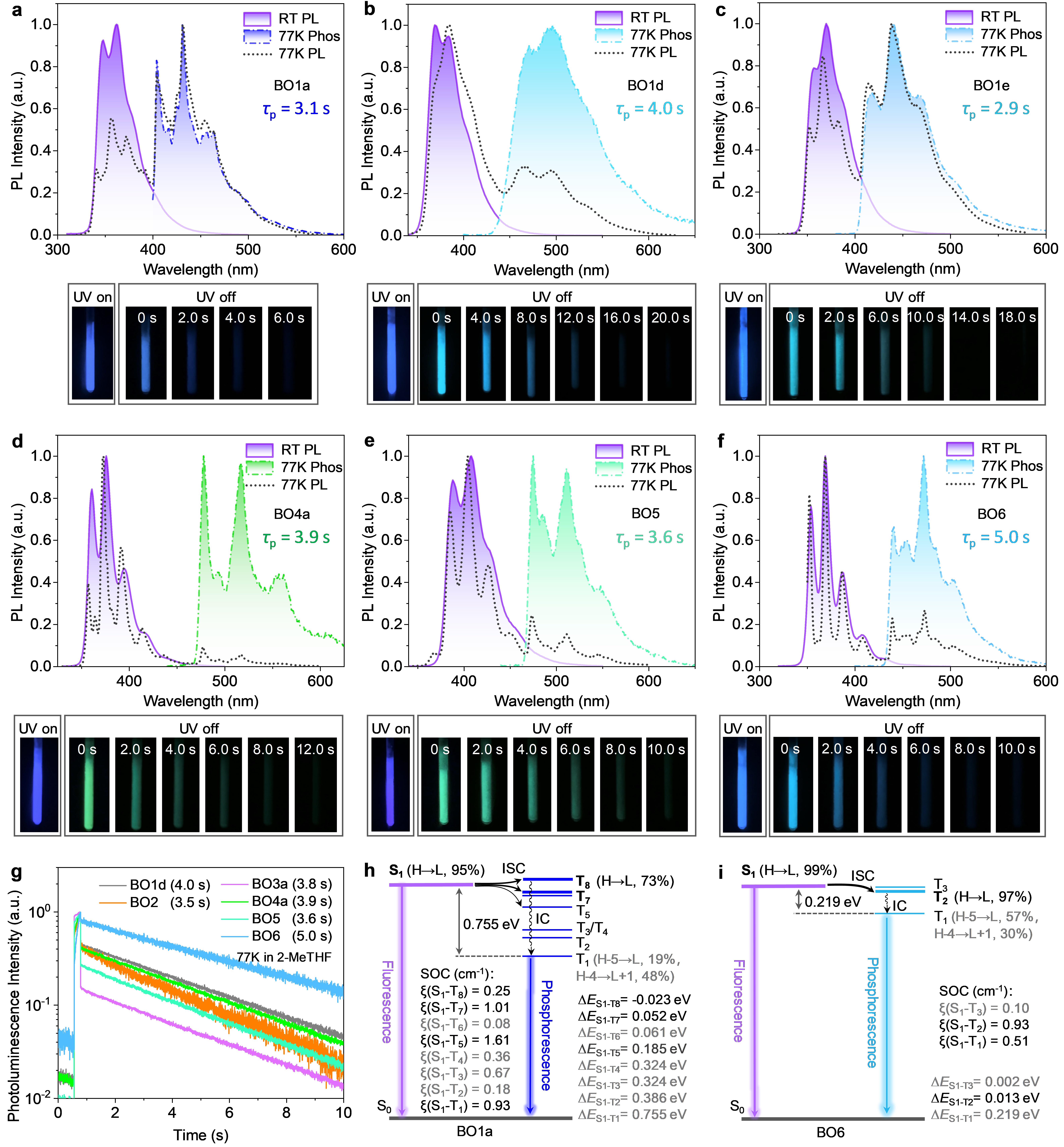
Fig. 3 Photophysical properties and state energy diagrams of dBO-PAHs. a–f Comparison of room-temperature PL spectra in dichloromethane, as well as 77 K PL and phosphorescent spectra in 2-MeTHF of BO1a, BO1d, BO1e, BO4a, BO5, and BO6, along with corresponding ultralong organic phosphorescence (UOP) photographs at 77 K in THF; the corresponding 77 K phosphorescent lifetime (τP) is shown in the inset. g Comparison of PL decay curves of BO1d, BO2, BO3a, BO4a, BO5, and BO6 at 77 K in 2-MeTHF upon excitation at their corresponding first phosphorescent peak. h,i TD-DFT-calculated singlet and triplet energy levels, and main transition configurations of BO1a and BO6 obtained at the B3LYP/6-31G(d) level based on the optimized S0 geometry; as well as spin-orbit coupling (SOC) matrix elements between S1 and Tn states of BO1a and BO6 evaluated using PySOC at the B3LYP/6-31G(d,p) level. RT, room temperature; PL, photoluminescence spectrum; Phos, phosphorescence spectrum; THF, tetrahydrofuran; 2-MeTHF, 2-methyltetrahydrofuran; ISC, intersystem crossing; IC, internal conversion; ξ, SOC value; ΔE, energy gap; H, HOMO; L, LUMO.
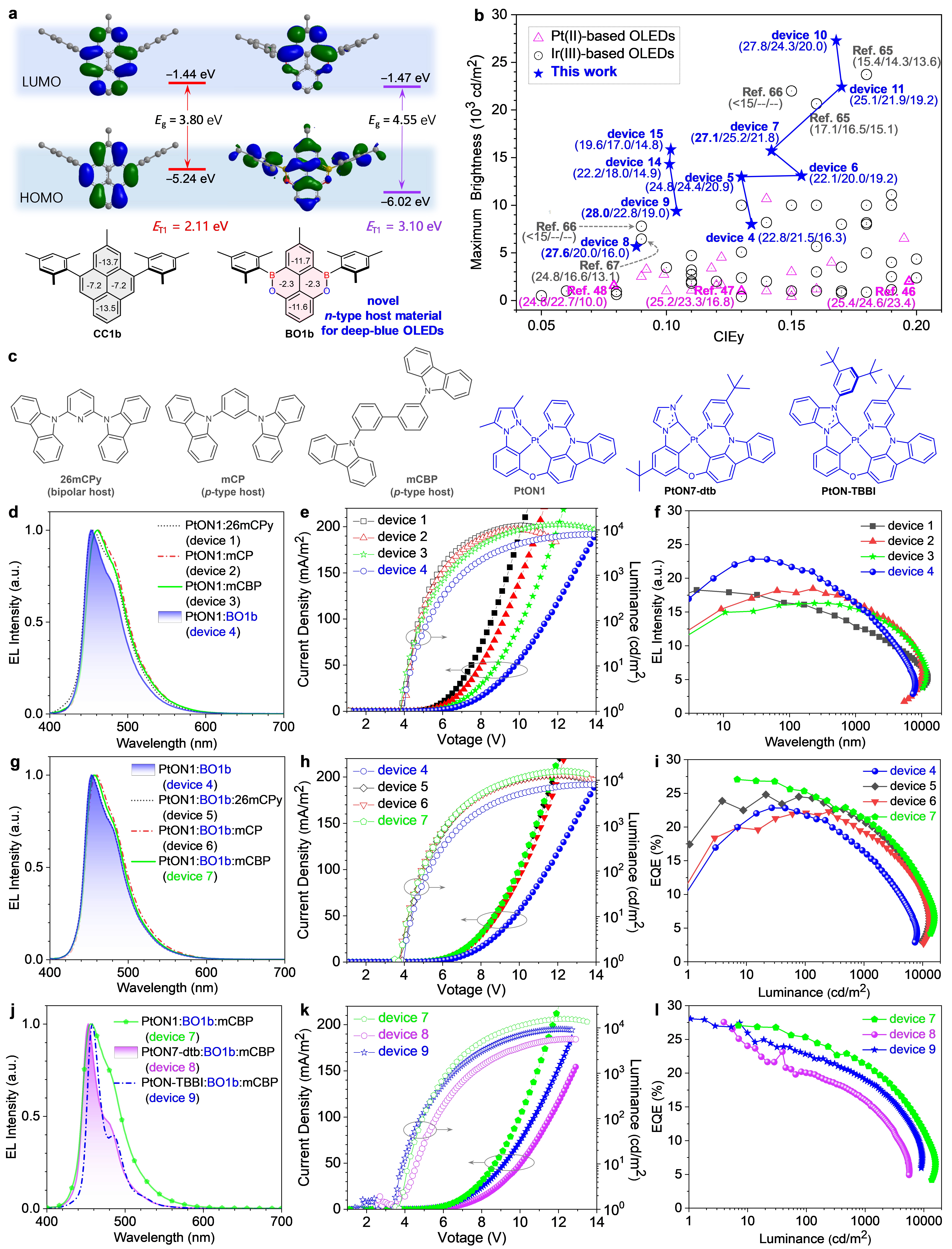
Fig. 4 Theoretical calculation, chemical structures and EL properties of deep-blue OLEDs. a Comparison of calculated frontier orbital distributions, energy levels/gaps, and NICS(1) values of BO1b and its carbon analogue CC1b. b CIEy vs. maximum brightness for Pt(II) and Ir(III)-based deep-blue OLEDs with CIEy < 0.20; devices connected with broken line to represent the devices with the same blue emitter; maximum EQE (%), and EQEs (%) at 100 and 1000 cd/m2 were provided in parentheses for representative deep-blue OLEDs. see Table S7 and Table S8 for detailed information. c, Chemical structures of host materials and Pt(II)-based deep-blue emitters used in this study. d,g,j EL spectra of deep-blue OLEDs at 1000 cd/m2. e,h,k Current density-voltage-luminance plots. f,i,l EQE vs. luminance plots. Eg, the energy gap between HOMO and LUMO; ET1, the energy level of the lowest triplet excited state; EL, electroluminescence spectrum; EQE, external quantum efficiency.
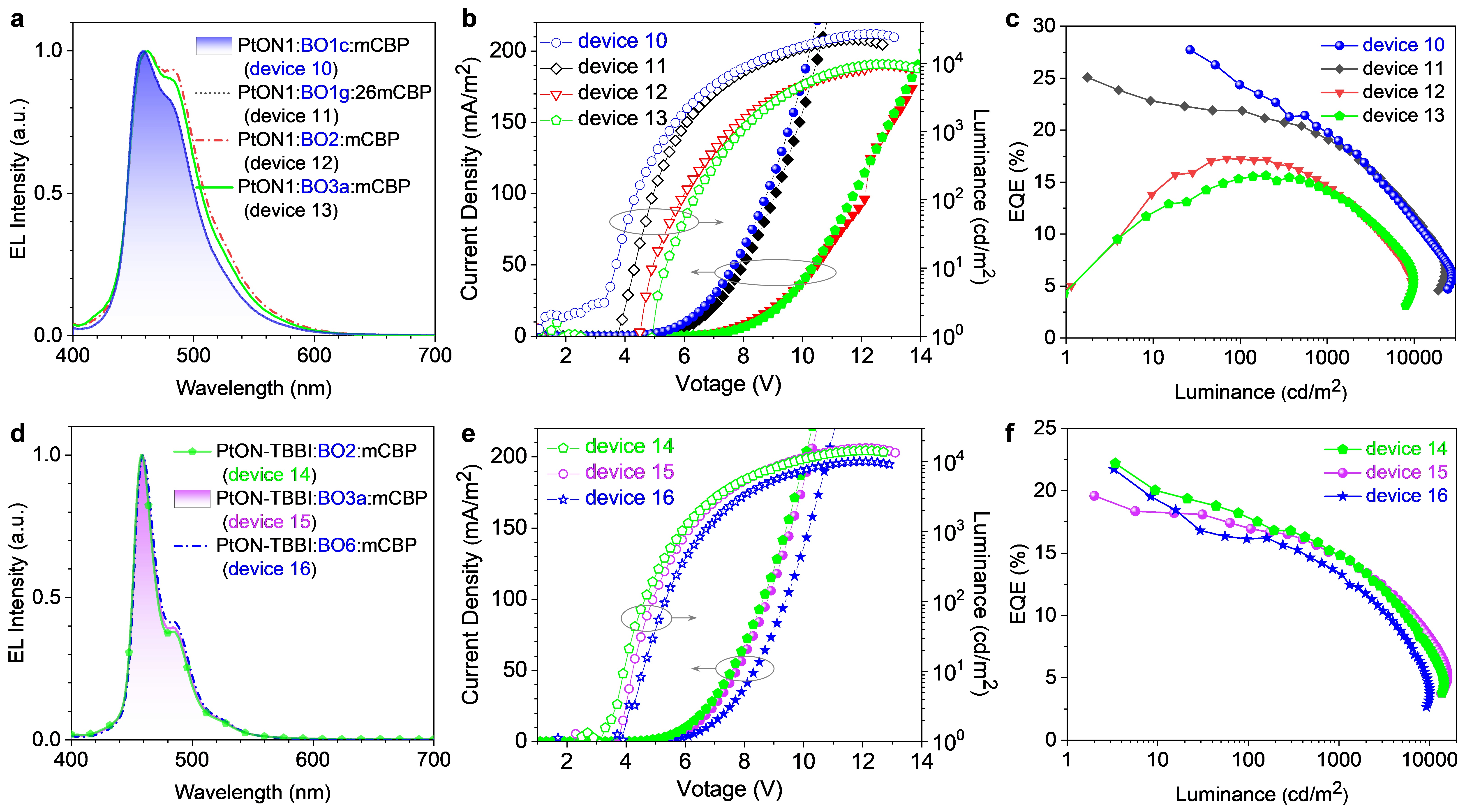
Fig. 5 EL properties of deep-blue OLEDs. a,d EL spectra of deep-blue OLEDs at 1000 cd/m2. b,e Current density-voltage-luminance plots. c,f EQE vs. luminance plots. EL, electroluminescence spectrum; EQE, external quantum efficiency.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in