
Propylene – the feedstock to produce polymers, oxygenates, personal care products, and detergents – is a key chemical building block having a global market size exceeding US$84 billion per year.1 In the past, propylene was produced mainly as a by-product of naphtha steam cracking for ethylene production. However, the shift to shale gas liquids as feedstocks for ethylene production has led to less propylene production via this route.2 The gap between propylene supply and ever-increasing demand is growing every year. To bridge the propylene gap,1,2 on-purpose technologies for propylene production are needed.
The CO2/CO electroreduction reaction (CO2RR/CORR) to valuable chemicals and fuels provides an attractive avenue to store intermittent renewable electricity.3 In CO2RR/CORR, among products reported from C1 to C3, n-propanol is detected as the main C3 product but – to date – this has been with low selectivity, limited production rates, and poor stability.
When renewable n-propanol is efficiently produced, n-propanol dehydration to propylene will become an on-purpose route to address the propylene gap.
Technoeconomic analysis (TEA) has demonstrated that a profitable CO2RR/CORR system requires a reaction rate above 100 mA cm-2.4,5 Until now, the best n-propanol Faradaic efficiency (FE) reported has been 18% when considering prior CORR/CO2RR systems exhibiting current densities greater than 100 mA cm-2.6-12 Focusing on CORR, we sought to promote selectivity and production rate of n-propanol electrosynthesis via catalyst design. Prior theoretical studies showed that C3 generation in CORR requires two consecutive C-C coupling steps: both C1-C1 and C1-C2 coupling, where C3 products compete with C2 products.13,14
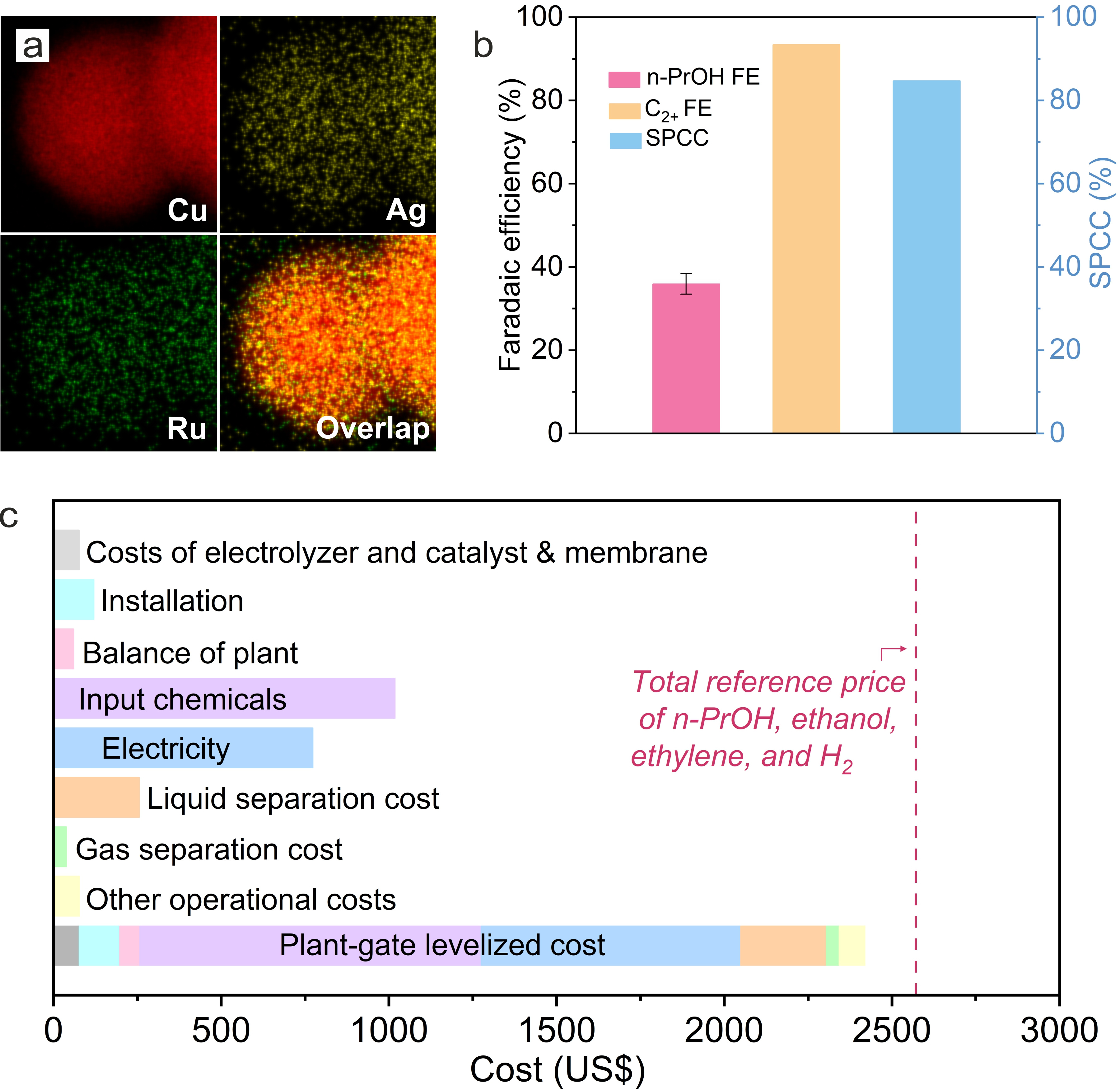
We took the view that, to improve C3 selectivity at a high production rate in CORR, the catalyst is expected to facilitate C1-C1 and C1-C2 coupling steps, stabilize C2 intermediates, and promote CO adsorption simultaneously. We developed a class of catalysts by doping Cu with Ag and Ru (Ag-Ru-Cu) through a two-step galvanic replacement approach (Figure a). In CORR, Ag-Ru-Cu catalysts increased selectivity toward both n-propanol and C2+ products, compared to Cu and Ag-Cu catalysts. Specifically, Ag-Ru-Cu catalysts delivered a n-propanol FE of (37 ± 3) % – more than 2× improvement versus the best prior n-propanol electrosynthesis above 100 mA cm-2 – at a production rate of (111 ± 9) mA cm-2, as well as 100-hour-stable n-propanol electrosynthesis at 300 mA cm-2.
With the aid of calculations and in situ/operando characterization (Raman and X-ray absorption), we found that, relative to Cu and Ag-Cu, the co-doping Ag and Ru into Cu lowers the activation energies for both C1-C1 and C1-C2 coupling, promotes CO adsorption, and stabilizes the key C2 intermediate (*OCCO) for C1-C2 coupling, thus promoting C3 selectivity.
We scaled the n-propanol electrosynthesis on Ag-Ru-Cu catalysts to a 15 cm2 membrane electrode assembly (MEA) electrolyzer. In this scaled demonstration, under the current density of 300 mA cm-2, we achieved a n-propanol FE of (36 ± 3) % and a C2+ FE of 93% at the full-cell potential of (-2.60 ± 0.02) V, together with a high single-pass CO conversion of 85% (Figure b) and full-cell energy efficiency of 37% for all C2+ products. Based on the above CORR performance in 15 cm2 MEA electrolyzer, our TEA calculation projects profitability: to produce one tonne of n-propanol plus the corresponding quantity of ethanol, ethylene, and H2 on Ag-Ru-Cu catalysts, the plant-gate levelized cost is projected to be lower than the sum of their reference prices (Figure c).
If you are interested in our work, you may find the full paper here: https://www.nature.com/articles/s41560-021-00967-7
References:
- Monai, M., Gambino, M., Wannakao, S. & Weckhuysen, B. M. Propane to olefins tandem catalysis: a selective route towards light olefins production. Soc. Rev. 50, 11503-11529 (2021).
- Akah, A. & Al-Ghrami, M. Maximizing propylene production via FCC technology. Petrochem. Res. 5, 377-392 (2015).
- Jhong, H.-R., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Opin. Chem. Eng. 2, 191-199 (2013).
- Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Eng. Chem. Res. 57, 2165-2177 (2018).
- Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Catal. 2, 1062-1070 (2019).
- Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Catal. 1, 748-755 (2018).
- Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Commun. 9, 4614 (2018).
- Zhuang, T.-T. et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Catal. 1, 946-951 (2018).
- Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Catal. 2, 251-258 (2019).
- García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661-666 (2020).
- Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Catal. 1, 421-428 (2018).
- Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 Nat. Catal. 1, 111-119 (2018).
- Xiao, H., Cheng, T. & Goddard, W. A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu (111). Am. Chem. Soc. 139, 130-136 (2016).
- Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050-7059 (2012).
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in