
Carbon dioxide electroreduction (CO2RR) powered by renewable-electricity to chemicals and fuels is a promising route to store the intermittent renewable energy.[1] Renewable methane (CH4) produced from CO2RR, a carbon-neutral alternative to fossil fuels, attracts interest in view of the well-established infrastructure for natural gas storage, distribution, and utilization.[2]
Prior progress in methane selectivity in CO2RR has mainly been made below current density 50 mA cm-2.[3-7] Technoeconomic analyses suggest that practical CO2RR systems require current density above 100 mA cm-2 to make systems profitable.[8] This motivated us to seek to increase, simultaneously, the current density and selectivity of methane production from CO2RR.
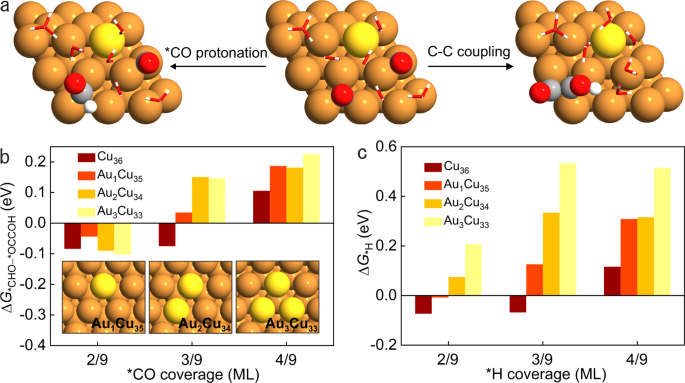
In CO2RR, after the generation of *CO intermediate, *CO protonation to *CHO is the potential-determining step for methane formation, competing with carbon-carbon (C-C) coupling for C2 products as well as the hydrogen evolution reaction (HER).[9-11] Thus, the key to improve methane selectivity is to suppress C-C coupling and HER simultaneously.
In a prior CO2RR study, we found that, on Cu surface, decreasing *CO coverage improved methane selectivity, but with prominent HER.[12] To circumvent the favorable HER and increase the selectivity to methane, we developed a suite of Au-Cu bimetallic catalysts and presented a strategy wherein we controlled *CO availability on Au-Cu catalysts, enabling selectivity to methane at high production rates in CO2RR.
We first fabricated Au-Cu catalysts, based on Cu catalysts supported on polytetrafluoroethylene (PTFE) nanofibers, using a galvanic replacement enabled by the differing reduction potentials of Au and Cu. Using this approach, we obtained a series of Au-Cu catalysts with different atomic percentages of Au in Cu.
In CO2RR, we regulated *CO availability on Au-Cu catalysts through controlling the CO2 concentration and reaction rate. By supplying gas streams consisting of different volume ratios of CO2 to N2, we evaluated CO2RR performance on Au-Cu catalysts under different current densities. Compared to pure CO2, the methane selectivity is promoted on Au-Cu catalysts in CO2–N2 mixed streams at high current densities while the selectivity to ethylene – the main C2 product – decreases dramatically. Relative to Cu catalysts, HER on Au-Cu catalysts is suppressed with increased methane selectivity under low *CO coverage; this leads to a 1.6× improvement in the methane:H2 selectivity ratio compared with prior reports[12-16] having a total current density above 100 mA cm-2. We as a result achieve a methane Faradaic efficiency (FE) of (56 ± 2) % at a partial current density of (112 ± 4) mA cm-2. With the aid of density functional theory calculations and operando X-ray absorption spectroscopy, we found that, under low *CO coverage, the introduction of Au in Cu favors *CO protonation vs. C−C coupling while suppresses HER.
These findings in this work suggest a promising strategy to directly convert dilute CO2 stream to carbon-neutral methane with a combination of high selectivity and high reaction rate.
If you are interested in our work, you may find the full paper here: https://www.nature.com/articles/s41467-021-23699-4
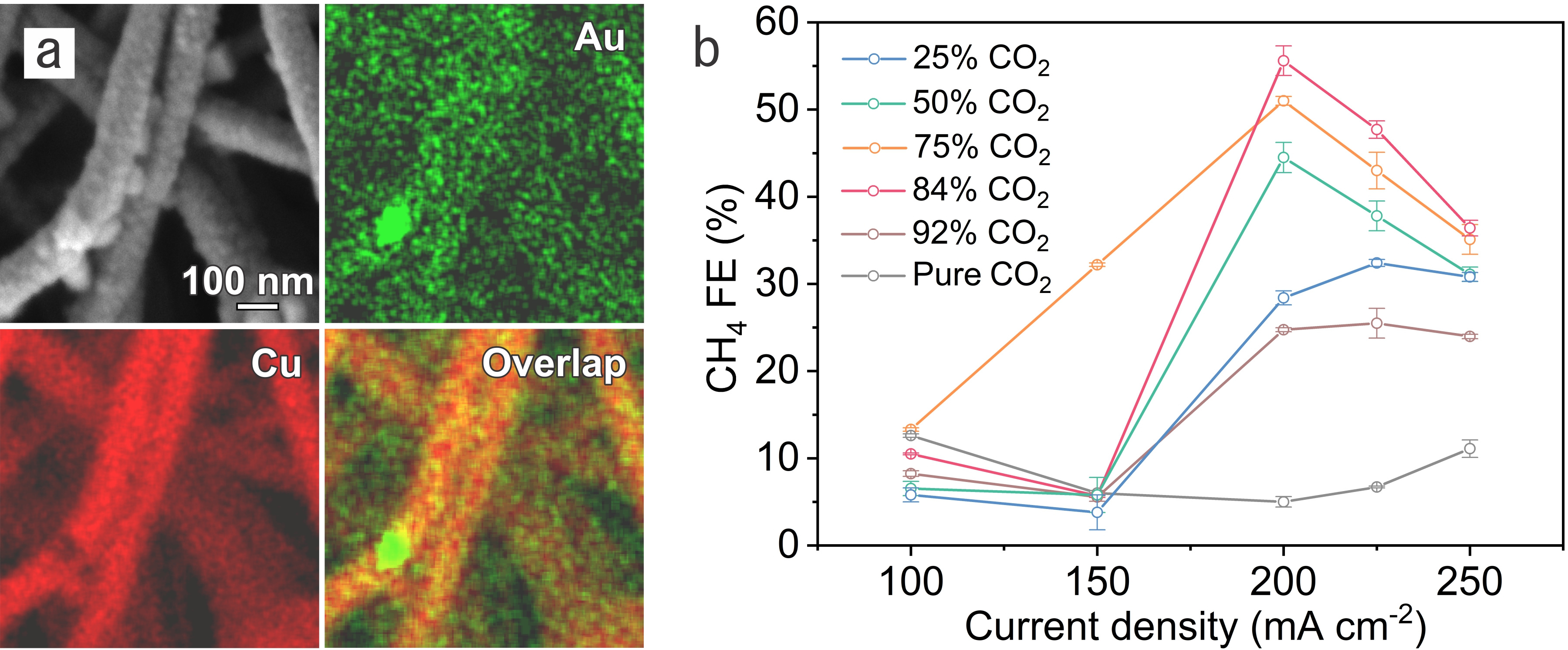
Figure. a, Secondary electron image and the corresponding energy-dispersive X-ray spectroscopy elemental mapping of Au and Cu for the 7% Au-Cu/PTFE catalyst. b, Methane FEs on 7% Au-Cu/PTFE at various CO2 concentrations.
References:
- Jhong, H.-R., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191-199 (2013).
- Howarth, R. W. & Ingraffea, A. Should fracking stop? Nature 477, 271-275 (2011).
- Qiu, Y.-L. et al. Copper electrode fabricated via pulse electrodeposition: toward high methane selectivity and activity for CO2 ACS Catal. 7, 6302-6310 (2017).
- Manthiram, K., Beberwyck, B. J. & Alivisatos, A. P. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 136, 13319-13325 (2014).
- Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312-1317 (2017).
- Reske, R., Mistry, H., Behafarid, F., Cuenya, B. R. & Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 136, 6978-6986 (2014).
- Zhao, H. et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane. Nat. Commun. 10, 3340 (2019).
- Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165-2177 (2018).
- Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Norskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311-1315 (2010).
- Cheng, T., Xiao, H. & Goddard, W. A. Free-energy barriers and reaction mechanisms for the electrochemical reduction of CO on the Cu(100) surface, including multiple layers of explicit solvent at pH 0. J. Phys. Chem. Lett. 6, 4767-4773 (2015).
- Gattrel, M., Gupta, N. & Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 594, 1-19 (2006).
- Wang, X. et al. Efficient methane electrosynthesis enabled by tuning local CO2 J. Am. Chem. Soc. 142, 3525-3531(2020).
- Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421-428 (2018).
- Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47-50 (2017).
- Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 Nat. Catal. 1, 111-119 (2018).
- Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584-8591 (2019).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in