Electrical Imaging: A new paradigm for generating high-dimensional functional data in live cells
Published in Chemistry, Physics, and Protocols & Methods

Cells process hundreds of input signals from the environment and respond through changes to gene expression, protein levels, functions, and processes. Two major challenges in drug discovery today are, 1) we do not understand all the variables at play that contribute to particular cellular phenotypes, and 2) most existing cell biology tools either measure a single functional readout or capture snap-shots at a single time-point, thereby limiting our ability to understand complex cellular responses. Removing these constraints, i.e. measuring many functional readouts over time, can help lead to better identification and classification of cellular phenotypes. For example, having more information during compound screening allows scientists to better understand how their prospective drugs are affecting live-cell biology, exposing off-target effects earlier in the process and enabling faster iterations on drug design.
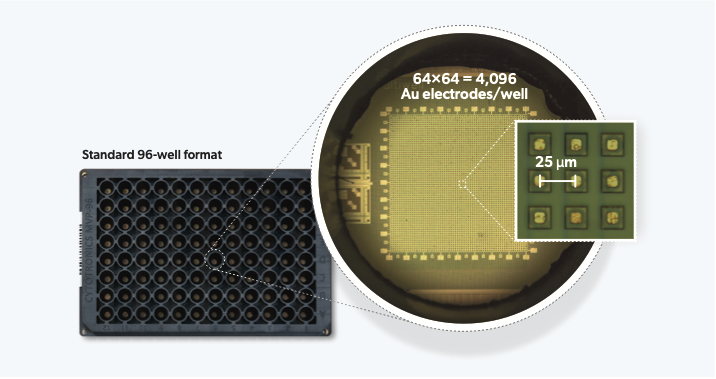
CytoTronics originated from research conducted at Harvard University, which combined semiconductor technology with biological applications. The core technology leverages the physical properties of cells and tissues which impede and distort electric fields in solution, predominantly due to lipid cell membranes which are poor conductors of electricity at low alternating current (AC) frequencies (<100 Hz) but can conduct at higher frequencies (>1 kHz). We therefore apply different electric field geometries at different (AC) frequencies to acquire a range of morphological and functional characteristics of live-cells. An exciting aspect of this technology is that it is completely non-destructive and label-free – allowing us to take measurements continuously over days and weeks without affecting cells. Furthermore, as all cells have membranes, the technique is versatile and can measure virtually any cell type. The use of semiconductor technology allows the creation of electrodes to apply these AC electric fields to be constructed at high spatial densities (e.g., 25 μm pitch/spacing) for close-to-single-cell resolutions – acquiring information beyond well-level population aggregates.
In our recently published study in Nature Communications, we integrate high-resolution semiconductor devices into a standard 96-well format and miniaturize off-plate electronics to allow measurement of up to 24 plates at the same time. This represents an engineering feat of exceptional scale – more than 2 orders of magnitude higher experiment throughput than any semiconductor-based biology experiment to date. The semiconductor 96-microplate platform features a 64 × 64 = 4096 electrode array at 25 μm at the bottom of each well for measurement. The ability to generate the single-cell resolution high-dimensional phenotypic datasets at scale, finally takes the semiconductor technology beyond the research lab, making it highly relevant for drug discovery applications.
Our unique measurements, using multiple field geometries and 4 different frequencies, measure 27 different impedance parameters over time. Based on our understanding of the measurements, we built hypotheses of the functional characteristics of cells that they measure. This was then tested and confirmed using a diverse set of cell-lines and compound treatments. We identified 5 measurements that clearly link to biological characteristics: tissue barrier, cell surface attachment, cell flatness, confluence and motility. The remaining measurements, which do not link directly to a single biological feature, are treated as high-dimensional parameters of cell-state.
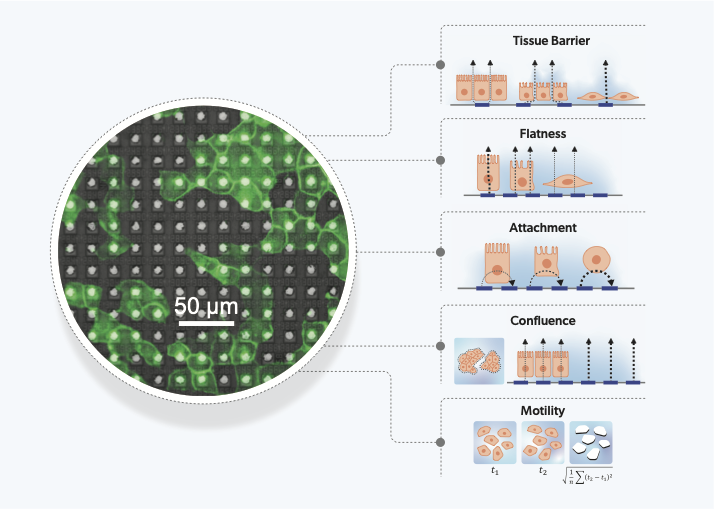
In characterizing 11 cell types with a range of properties, we found many observations matched known behaviors, e.g.: epithelial cell types had a high tissue barrier compared to cancer cells, and mesenchymal cancer cells demonstrated lower attachment and higher motility compared to those that were more epithelial-like. The platform’s spatial resolution was then shown to identify populations of two distinct cell types in the same culture, demonstrated by a co-culture of epithelial (MCF7) and mesenchymal-like (MDA-MB-231) breast cancer cells for application in epithelial-to-mesenchymal transition (EMT) research. The ability to distinguish and identify heterogeneous cell populations, enabled by the thousands of electrode sensors per well at single-cell resolutions, allows our tool to be applied to more physiologically relevant systems that include multiple cell-types.
Live-cell electrical imaging of functional phenotypes of 11 cell lines
Cells were plated at a range of densities laid out from low to high, top to bottom in each column for the indicated cell type. Videos were generated with VF 250 Hz in red, VF 16 kHz in green, and the inverse of the LF 16 kHz in blue. Time ranges from 0-48 hours post seeding.
Finally, we performed a compound screen on A549 cells using 904 compounds applied across 13 semiconductor 96-microplates. With the rising popularity of Cell Painting and other high content imaging based approaches, our platform’s ability to capture high-dimensional functional data is a very complementary approach. Our technology can capture the entire temporal kinetics for a compound response across multiple unique functional readouts – creating many layers of dimensionality compared to an end-point readout. In analyzing our high dimensional data, we identified 25 unique clusters based on cell response, and could even distinguish between closely related mechanisms of action (MOA). For example, we identified 2 DNA damage clusters governed by distinct mechanisms of DNA synthesis inhibition and double-stranded break formation. Distinguishing between compound MOAs by kinetics and intermediate effects on cell morphology can greatly aid drug development processes which aim to optimize for specific MOAs and driving out off-target effects.
Our newly developed semiconductor platform captures more than 10× information per biological sample compared to previous commercial techniques, at a scale of throughput useful for early drug discovery screening. Altogether, it creates an information rich map of live-cell function over time at unprecedented resolution. At CytoTronics, we are bringing our technology to market in early 2024, which will make it possible for any researcher to conduct their own semiconductor based experiments in their lab. Furthermore, our future plans include maximizing the utility of the electrodes and integrated circuits to expand functionalities – measuring the activity of electrogenic cells such as cardiac and neural cells and redox potential/metabolism levels. Additionally, our electrode array can also be configured for cell stimulation and manipulation – for example, selectively wounding a tissue or electrically stimulating electrogenic cells. These expanded platform capabilities will enable more diverse applications for pharmaceutical and cell-biology research.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in