Electrosynthesis of buckyballs with fused-ring systems from PCBM and its analogue

The main research interests of the Wang group at University of Science and Technology of China are fullerene chemistry (Chem. Soc. Rev., 2013, 42, 7535) and green organic synthesis (Chem. Soc. Rev., 2013, 42, 7668). The recent advances in fullerene chemistry mainly include electrochemical reactions of fullerenes (Angew. Chem. Int. Ed., 2014, 53, 3006; Sci. Bull., 2022, 67, 2406; Angew. Chem. Int. Ed., 2023, 62, e202304321) and cage modifications of endohedral metallofullerenes (Angew. Chem. Int. Ed., 2011, 50, 4658; J. Am. Chem. Soc., 2012, 134, 29, 11956; Angew. Chem. Int. Ed., 2023, 62, e202313074). More recently, we have also applied fullerene derivatives prepared by the synthetic methodology developed by our group to organic photovoltaics (OPVs) and perovskite solar cells (PSCs), with an effort to identify functional fullerene compounds with excellent power conversion efficiencies (Sci. Bull., 2022, 67, 2406; Angew. Chem. Int. Ed., 2023, 62, e202304321; Angew. Chem. Int. Ed., 2023, 62, e202313074).
Esters are ubiquitous and an important class of organic compounds, which have been widely applied in the chemical and pharmaceutical industries, perfumery and cosmetics, and serve as precursors for many other types of organic molecules. Esters can undergo various functional group interconversions. However, the α-C−H functionalizations of esters via a radical process are rarely investigated. On the other hand, [6,6]-phenyl-C61-butyric acid methyl ester (PCBM, 1), a star molecule in fullerene chemistry and materials science, has been widely used in solar cell devices1−5 due to its unique advantages in excellent solubility and electronic transmission capability. Derivatives of PCBM have also been synthesized and utilized in OPVs1,6,7 and PSCs.2−5,7 Among them, PCBM(CH2) was one of the most representative examples. Taking the previous complex synthetic steps for an isomeric mixture of PCBM(CH2)8 into consideration and in continuation of our interest in the electrosynthesis of fullerene derivatives (Angew. Chem. Int. Ed., 2014, 53, 3006; Chem. Sci., 2019, 10, 3012; Chem. Sci., 2020, 11, 384; Research, 2020, 2020, 2059190; Sci. Bull., 2022, 67, 2406; Angew. Chem. Int. Ed., 2023, 62, e202304321), we anticipated that the straightforward cyclopropanation of the electrochemically generated dianion of PCBM with CH2I2 via dual nucleophilic reactions would regioselectively afford a single isomer of PCBM(CH2). Surprisingly, CH2I2 was not involved, and two classes of buckyballs with fused bi- and tricyclic carbocycles via intramolecular radical α-C−H functionalization of the ester moiety were isolated for both PCBM and its analogue, i.e., [6,6]-phenyl-C61-propionic acid methyl ester (PCPM, 2).9 Recently, our paper published on Nature Communications described the details of these intramolecular annulations of the electrochemically generated dianions of PCBM and PCPM in the presence of a trace amount of oxygen, and preliminarily investigated the application of a representative fullerene derivative in inverted planar PSC devices.
In this manuscript, we report that two classes of buckyballs with fused bicycles or tricycles can be electrochemically synthesized (Fig. 1). Furthermore, an unknown type of bisfulleroid 8 can be efficiently obtained from PCPM. Intriguingly, bisfulleroid 8 has two tethered orifices at [6,6]-bonds with a 14-membered-ring opening instead of the previous common bisfulleroids at [5,6]-bonds,10 exhibiting a unique addition pattern for open-cage fullerene derivatives. The chemical structures for all three types of products have been established by single-crystal X-ray crystallography.
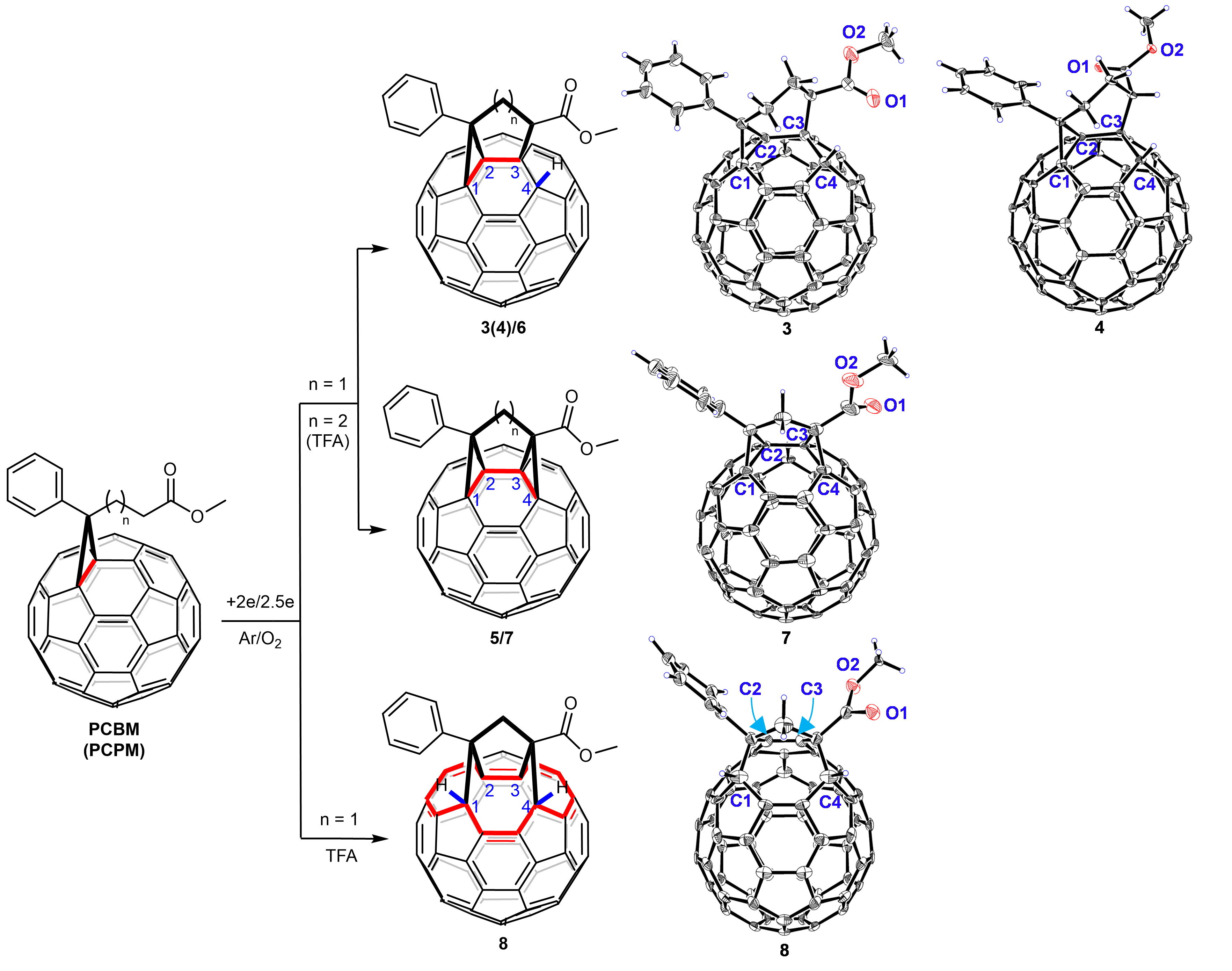
Fig. 1 | Electrosynthesis of buckyballs and Oak Ridge thermal ellipsoid plot (ORTEP) diagrams. ORTEP diagrams of 3, 4, 7 and 8 with 20%, 20%, 10% and 20% thermal ellipsoids, respectively. The solvent and decapyrrylcorannulene molecules are omitted for clarity. The red color indicates the characteristic addition site for the fused ring system or the 14-membered-ring opening.
An intramolecularly annulated isomer of PCBM, 3, has been applied as an additive to inverted planar perovskite solar cells (Fig. 2), and the power conversion efficiency increases significantly from 15.83% to 17.67%, demonstrating its enormous potential in solar cell devices.
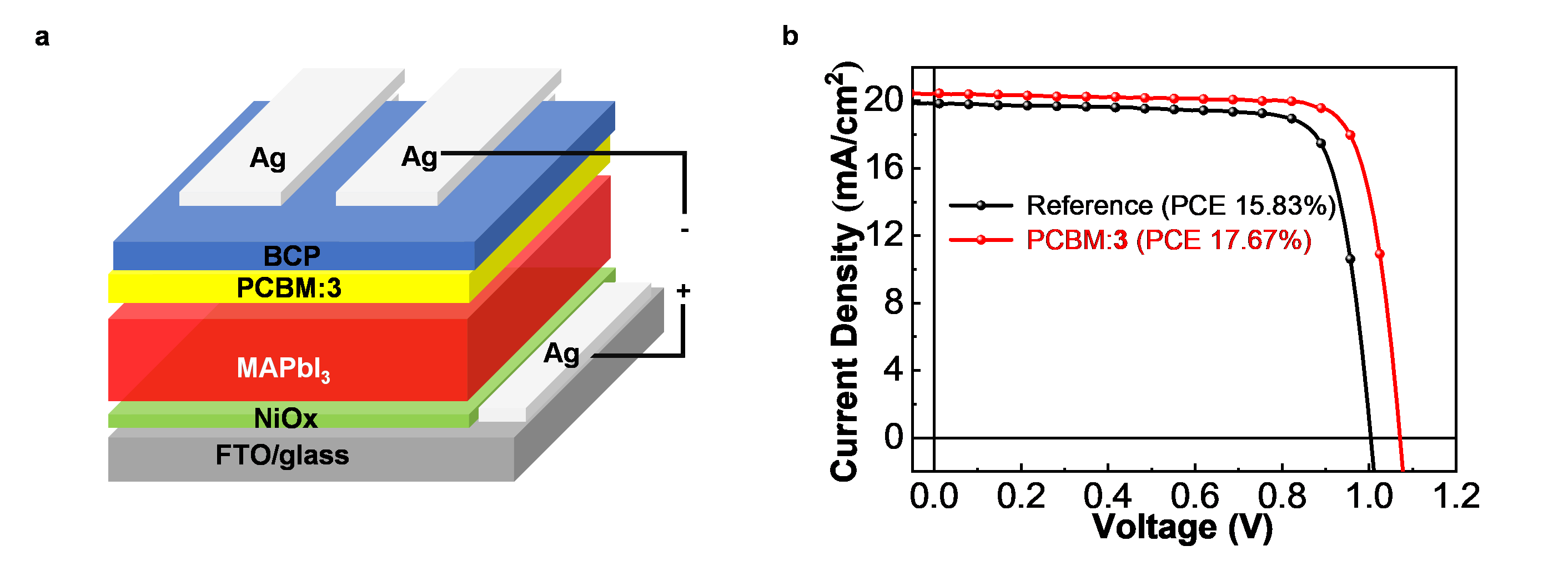
Fig. 2 | Application to perovskite solar cells. a Illustration of the inverted planar perovskite solar cell structure used in this work. b J–V curves (reverse scan) of the device with PCBM:3 (red line) and the reference device with PCBM (black line). BCP: bathocuproine, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline. PCBM: [6,6]-phenyl-C61-butyric acid methyl ester. MA: methylammonium. FTO: fluorine doped tin oxide.
For more details of this work, please see our paper titled “Electrosynthesis of buckyballs with fused-ring systems from PCBM and its analogue” in Nature Communications.
References
- Li, C.-Z., Yip, H.-L. & Jen, A. K.-Y. Functional fullerenes for organic photovoltaics. Mater. Chem. 22, 4161−4177 (2012).
- Fang, Y., Bi, C., Wang, D. & Huang, J. The functions of fullerenes in hybrid perovskite solar cells. Adv. Energy Mater. 2, 782–794 (2017).
- Jia, L., Chen, M. & Yang, S. Functionalization of fullerene materials toward applications in perovskite solar cells. Chem. Front. 4, 2256–2282 (2020).
- Liu, K., Tian, C., Liang, Y., Luo, Y., Xie, L. & Wei, Z. Progress toward understanding the fullerene-related chemical interactions in perovskite solar cells. Nano Res. 15, 7139–7153 (2022).
- Xing, Z., Li, S.-H. & Yang, S. Targeted molecular design of functionalized fullerenes for high‐performance and stable perovskite solar cells. Small Struct. 3, 2200012 (2022).
- Lai, Y.-Y., Cheng, Y.-J. & Hsu, C.-S. Applications of functional fullerene materials in polymer solar cells. Energy Environ. Sci. 7, 1866–1883 (2014).
- Umeyama, T. & Imahori, H. Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Chem. Res. 52, 2046−2055 (2019).
- Li, C.-Z., Chien, S.-C., Yip, H.-L., Chueh, C.-C., Chen, F.-C., Matsuo, Y., Nakamura, E. & Jen, A. K.-Y. Facile synthesis of a 56π-electron 1,2-dihydromethano-[60]PCBM and its application for thermally stable polymer solar cells. Commun. 47, 10082–10084 (2011).
- Zhao, G., He, Y., Xu, Z., Hou, J., Zhang, M., Min, J., Chen, H.-Y., Ye, M., Hong, Z., Yang, Y. & Li, Y. Effect of carbon chain length in the substituent of PCBM-like molecules on their photovoltaic properties. Funct. Mater. 20, 1480–1487 (2010).
- Cerón, M. R., Izquierdo, M., Aghabali, A., Valdez, J. A., Ghiassi, K. B., Olmstead, M. M., Balch, A. L., Wudl, F. & Echegoyen, L. Tethered bisadducts of C60 and C70 with addends on a common hexagonal face and a 12-membered hole in the fullerene cage. Am. Chem. Soc. 137, 7502−7508 (2015).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in