Eliminating cell walls: dead or alive?
Published in Microbiology

Since the discovery of L-form bacteria back to the 1930s there have been many reports on the possible role of L-forms in chronic or recurrent infections, but the molecular events underlying switching to and from the L-form state were poorly understood. In the mid-2000s, the Errington group started to tackle this long-standing problem. Early work resulted in the development of an inducible Bacillus subtilis L-form system in a highly efficient and reproducible manner, enabling us to study the cell biology and genetics of L-forms. The first remarkable finding was that the FtsZ-based division machine, required for cell division in most bacteria, became dispensable in L-forms for their proliferation (Leaver et al., Nature 2009). This report spurned a lot of interest in L-forms, not least for the possibility that they might exemplify how primordial cells proliferated way back before the cell wall evolved more than 3 billion years ago.
In 2011, I joined this L-form project to explore the molecular mechanisms of FtsZ-independent L-form growth. We wondered why simple cell wall-free protoplasts, induced by lysozyme treatment, are unable to divide. To answer this question, we carried out extensive genetic screens to isolate mutants enabling L-form development from non-growing protoplasts. After a couple of years of work, a quite simple conclusion emerged, that the combination of two kinds of mutations was sufficient to promote L-form growth in protoplasts. The class 1 mutations worked to promote L-form division by generating excess amounts of cell membrane (Mercier et al., Cell 2013). The other class of mutations seemed to work to avoid toxic reactive oxygen species (ROS) generation from oxygenic respiration (Kawai et al., Curr. Biol. 2015). Our new paper published in Nature Microbiology shows that cell wall inhibition in L-forms causes a metabolic diversion, resulting in the ROS-mediated cell death through increased glycolytic flux (https://www.nature.com/articles/s41564-019-0497-3). The results gain in importance because they also help to explain molecular details of how cell-wall targeting antibiotics, such as penicillins, actually kill bacteria.
In the course of the research we realised that our results would be much more convincing if we could back up the conclusions from our genetic and physiological experiments with direct measurements of key metabolites in the cell. We were fortunate that my supervisor, Jeff Errington, gave a seminar at the Crick Institute in London on the invitation of Dr Luiz Pedro Sório de Carvalho (Luiz). Following this we established a very fruitful collaboration. I visited the Crick to work with Luiz’ post-doc, Dr Agnese Serafini, to initiate the experiments that led to the critical supporting data being obtained.
We now have a much better understanding of what are needed to enable bacteria to grow without the need for a cell wall or FtsZ under laboratory conditions (Figure 1). L-forms can escape from the surrounding cell wall by lysozyme treatment in osmoprotective media (Kawai et al., Cell 2018). Inhibition of cell wall synthesis promotes excess membrane generation, which drives the proliferation of L-forms (Mercier et al., Cell 2013). Avoidance of ROS toxicity by compensating for the metabolic imbalance in L-forms leads to their robust growth. Those requirements also work to promote L-form generation in many Gram-positive bacteria, including important pathogens.
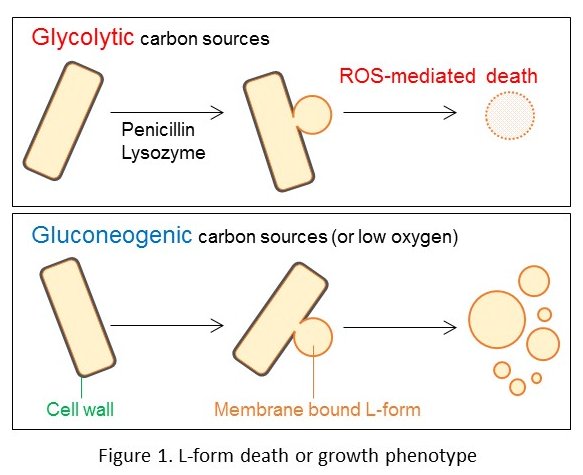
I think that switching to the L-form state is an important illustration that essentiality or non-essentiality in biological pathways is critically dependent on the environment in which the bacterium lives. L-forms may form part of the “unculturable” bacteria thought to be abundant in nature, including the unidentified survivors of antibiotic treatment in human patients.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in