Engineering biomimetic vascular networks
Published in Bioengineering & Biotechnology

One of our favorite examples of science-as-art is the vascular corrosion cast: a physical replica of an explanted organ’s internal vascular networks made by first injecting the vasculature with plastic resin, then chemically degrading the original tissue (Fig. 1). These captivating structures remind us of the spectacular architecture woven throughout nature, but in our research group, they also serve as goalposts for our ongoing efforts in vascular biofabrication.
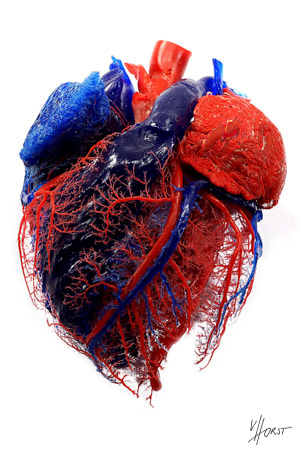
Highly branched, tree-like vascular networks, as showcased by these corrosion casts, are widespread because they are very efficient at transporting oxygen and nutrients to cells; for the same reason, our field has long been interested in incorporating such networks into engineered tissues. Actually making these networks in vitro presents a pair of questions: first, how to design the blueprints for networks with these complex branching features; and second, how to physically produce them.
To address the design question, we collaborated with Jessica Rosenkrantz and Jesse Louis-Rosenberg, co-founders of the design studio Nervous System. Nervous System specializes in the design of organic structures by developing computer simulations of biological growth and pattern formation. By day, they use these algorithms to craft artwork, jewelry, puzzles, and more. To adapt these models to make branching networks for perfusable tissues, Jesse and Jessica began with one of their existing growth simulations, which uses a biophysics model to “grow” patterns reminiscent of leaf venation (Fig. 2). To make networks with a single inlet and outlet, they devised a modified algorithm where two or more trees grow towards one another and connect in the center of a growth domain – we call this process “mutual tree attraction”, and the resulting networks “dendritic networks” (Movie 1).
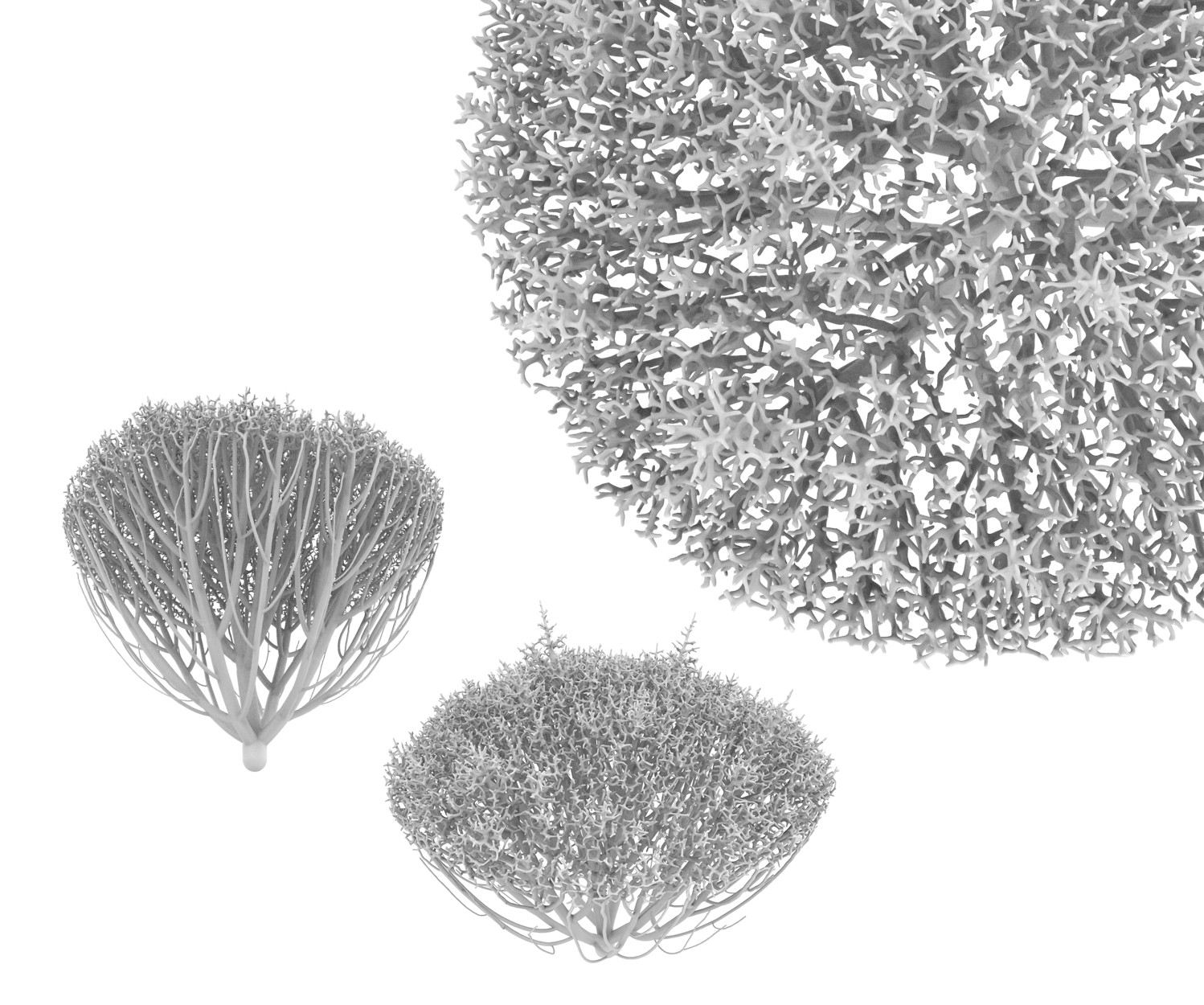
Under the hood, these dendritic networks are still based on development of leaf venation; however, the underlying similarity of the networks suggested to us that they could be a suitable starting point for engineered mammalian tissue. Generative design is incredibly valuable for engineering vasculature because it allows us to fill tissues of many shapes and sizes with vascular networks and parametrically tune the branching pattern.

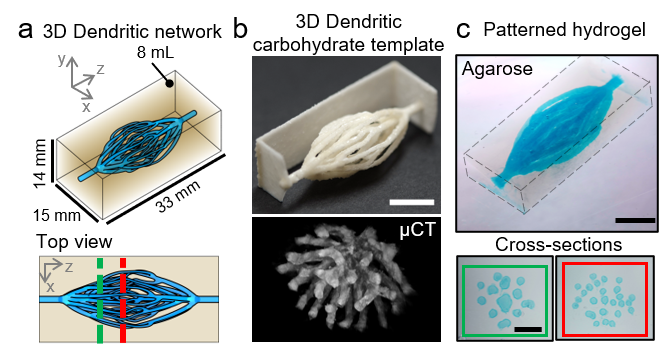
Reproducing these dendritic vascular networks within engineered tissues posed a major fabrication challenge. Our lab previously engineered vascular networks by making sacrificial templates out of 3D printed sugar glass [1], assembling tissues around the templates, and selectively removing the templates to leave an open channel network. The sacrificial strategy remained appealing, but we needed a more advanced way to 3D print sugar into the new network designs. Our solution was to extend the methodology of selective laser sintering [2] to be compatible with carbohydrate materials. The benefits of laser sintering – namely, a fully-supported, powder-based build volume – provided the necessary architectural freedom to print the dendritic networks. Our sacrificial approach allowed us to pattern the networks in a wide range of extracellular matrix materials with relatively high throughput (Fig. 3).
We explored these dendritic networks from several angles in this work, including measuring fluid flow through the networks and seeding endothelial and parenchymal cells. We became especially interested in mapping the spatiotemporal dynamics of cell metabolism and proliferation as a function of vascular proximity, and we observed intriguing patterns of gradient steepening which may emerge in the tissue lying outside a perfused vessel [3]. Ultimately, however, we were interested in evaluating the extent to which physiologically relevant, and highly metabolic, tissues could be supported by dendritic vascular networks.
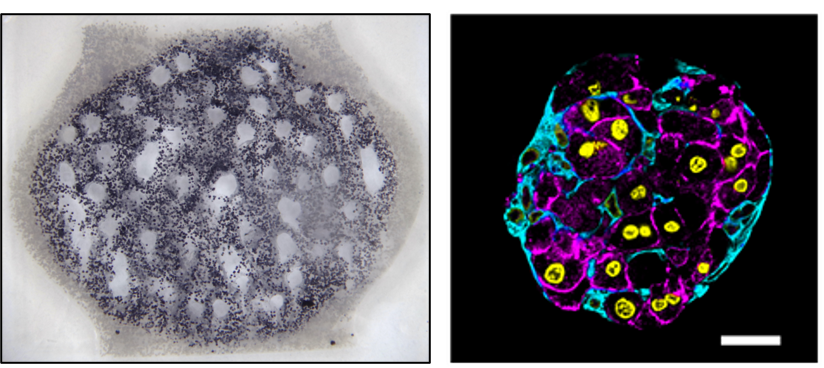
We worked with Kelly Stevens’ group (University of Washington Bioengineering) to study the survival and function of hepatic aggregates in our perfused tissues. Hepatic aggregates combine rodent hepatocytes with cultured fibroblasts as a simple but effective strategy to mitigate the rapid loss of liver-specific function that is otherwise seen when hepatocytes are cultured outside the body [4]. We were excited to observe that perfusion culture through dendritic networks can support metabolic function in thick tissues containing these aggregates, and also maintain a healthy aggregate morphology along with albumin production (Fig. 4).
Many challenges remain in the ongoing effort to engineer functional tissue substitutes, but we believe that these new strategies for designing and fabricating biomimetic networks will play a major role in pushing regenerative medicine to the whole-organ scale.
References
[1] J. S. Miller et al., “Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues,” Nat. Mater., vol. 11, no. 9, pp. 768–774, 2012. https://dx.doi.org/10.1038%2Fnmat3357
[2] I. S. Kinstlinger et al., “Open-source Selective Laser Sintering (OpenSLS) of nylon and biocompatible polycaprolactone,” PLoS One, vol. 11, no. 2, p. e0147399, 2016. https://doi.org/10.1371/journal.pone.0147399
[3] I. S. Kinstlinger et al., “Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates,” Nat. Biomed. Eng., 2020. https://doi.org/10.1038/s41551-020-0566-1
[4] K. R. Stevens et al., “InVERT molding for scalable control of tissue microarchitecture.,” Nat. Commun., vol. 4, no. May, p. 1847, 2013. https://dx.doi.org/10.1038%2Fncomms2853
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biosensing
Publishing Model: Hybrid
Deadline: Mar 26, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in