Engineering tumor microenvironments to study prostate cancer metastasis to bone
Published in Cancer
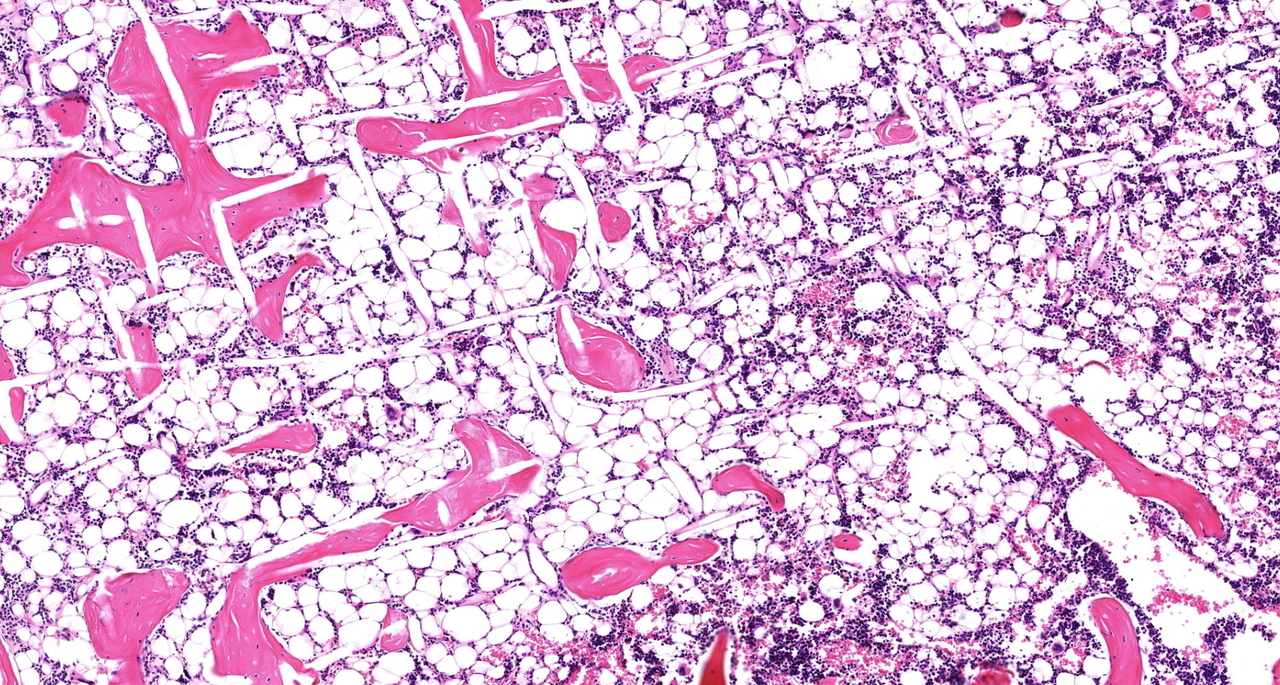
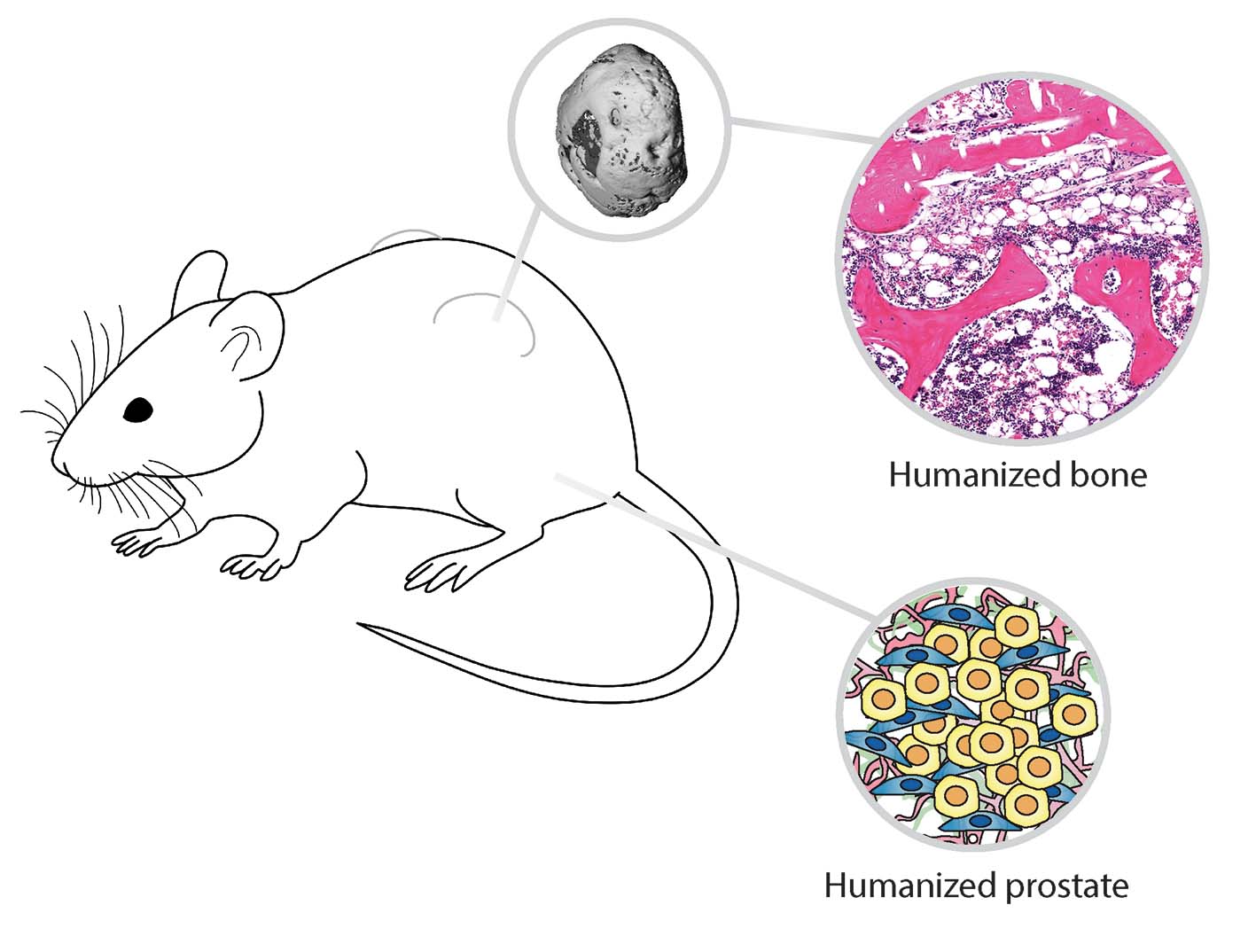
In our recent Communications Biology manuscript, we investigated the role the microenvironment plays in shaping tumor growth and subsequent bone metastasis. Harnessing tissue engineering techniques, we generated two tiers of humanization within an immunocompromised murine model: the primary prostate tumor with TME, and the bone organ as a metastatic site. Using androgen-sensitive human prostate adenocarcinoma cells derived from the left supraclavicular lymph node metastasis from a 50-year-old male (LNCaP) and an androgen insensitive cell line (PC-3) which was established in 1979 from bone metastasis of grade IV of prostate cancer in a 62-year-old patient. We demonstrated that the TME influences bone tropism in a manner specific to the cells with low (LNCAP) and high (PC-3) metastatic potential.
Project motivation
Traditional approaches to xenograft models center around studying the interaction of human cancer cells within a murine host, and thus murine microenvironments. While these xenografts have generated valuable results, current models lack important species-specific crosstalk events between the tumor and its host microenvironment at both the primary and metastatic sites. To overcome these limitations, our research program constructs and comprehensively characterizes state of the art experimental models to study the interaction of cancer cells and the bone metastatic microenvironment to better understand these complex interactions. By converging tissue engineering and regenerative medicine principles, we have created anatomical and functional humanized bone that recapitulates native human physiology1 and serves as a metastatic niche for bone-tropic tumors2-4.
The primary TME is an agent for prostate cancer development, growth, progression, and metastasis. However, the TME is also a complex and multifaceted entity and there is great difficulty in delineating the contributing roles of specific TME components in this context. To build on our extensive program engineering bone microenvironments to study cancer cell bone tropism, our team engineered a primary prostate tumor using prostate cancer cell lines and two critical TME cell types: cancer-associated fibroblasts (CAFs) and microvascular endothelial cells (MVECs). Both CAFs and MVECs have been implicated in directly contributing to prostate cancer metastasis5,6.
Concept development
Our team initially hypothesized that a humanized prostate cancer TME would enhance tumor growth and subsequent metastasis to bone and visceral organs. We postulated that interaction between human TME and human cancer cells would enhance prostate cancer metastasis in a species-specific manner. In our initial manuscript using the PC-3 cell line3 we were surprised to discover that this was not the case. Rather we reported that a prostate TME humanized with CAFs and MVECs specifically decreased PC-3 metastasis to the humanized bone niche, but not the murine skeleton3.
The current study
We next hypothesized that the rapid 4-week growth and metastasis of the PC-3 cell line did not allow for adequate interaction of the prostate cancer cells with the TME. Our team proposed that slower growing prostate cancer cells would more accurately reflect a primary tumor in crosstalk with and influenced by its associated TME. We also subsequently screened the PC-3 cell lines within our laboratory to assess growth rates and found heterogeneity within the lines. Interestingly, many still consider that cell lines are a reproducible and standardized model for in vitro and in vivo studies. A literature review revealed that genetic diversification, phenotypic and functional variations are observed in many other commonly used cell lines7. We next compared PC-3 cell lines from our laboratory and other sources and observed variations in proliferation rates, despite no differentiation upon cell line authentication.
In our current manuscript published in Communications Biology, we created a humanized prostate TME consisting of either the LNCaP or PC-3 prostate cancer cell line grown together with CAFs and MVECs in an orthotopic environment. Both the LNCaP- and PC-3-derived tumors developed and metastasized over a 10-11-week period. Interestingly, the humanized TME showed a trend to decrease metastasis of PC-3 cells to humanized bone but did not influence metastasis to the murine bones or visceral organs. In contrast, the humanized TME enhanced LNCaP primary tumor growth and bone metastasis. Our exciting results uncovers a potential new role of the TME in prostate cancer osteotropism which warrants further investigation.
Future directions
Our team aspires to provide exciting insights of the mechanistic events within the TME that influences prostate cancer bone tropism. Our original humanized rodent models based on more than two decades of bone tissue engineering research contribute to dissecting the nature of bone metastases. Yet, the degree to which these species-specific signaling processes can structure tumor cell communities is only beginning to be explored. Understanding the interaction between cancer cells and the TME may shed light on the propensity of certain cancers to metastasize to specific organ niches, such as prostate cancer metastasis to bone. Ultimately, this new knowledge will lead to the development of more effective therapeutic interventions to prevent the metastatic spread of cancer cells.
You can access our Communications Biology paper here
References
1. Martine, L. C. et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat Protoc 12, 639-663, doi:10.1038/nprot.2017.002 (2017).
2. Holzapfel, B. M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108-4115, doi:10.1016/j.biomaterials.2014.01.062 (2014).
3. McGovern, J. A. et al. Humanization of the prostate microenvironment reduces homing of PC3 prostate cancer cells to human tissue-engineered bone. Cancers (Basel) 10, doi:10.3390/cancers10110438 (2018).
4. Shafiee, A. et al. Immune system augmentation via humanization using stem/progenitor cells and bioengineering in a breast cancer model study. Int J Cancer 143, 1470-1482, doi:10.1002/ijc.31528 (2018).
5. Olumi, A. F. et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer research 59, 5002-5011 (1999).
6. Zeng, Y., Opeskin, K., Goad, J. & Williams, E. D. Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer research 66, 9566-9575, doi:10.1158/0008-5472.CAN-06-1488 (2006).
7. Ben-David, U. et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560, 325-330, doi:10.1038/s41586-018-0409-3 (2018).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in