Enhancement of Li+ Transport Through Intermediate Phase in High-Content Inorganic Composite Quasi-Solid-State Electrolytes
Quasi-solid-state electrolytes promise the safety of ceramics, the flexibility of polymers, and the conductivity of liquids—yet the “how” behind their superior ion transport has remained murky. Now, a joint team from Fudan University and the National Institute for Cryogenic & Isotopic Technologies (Romania), led by Professors Aishui Yu and Tao Huang, delivers a decisive answer in Nano-Micro Letters. Their review, “Enhancement of Li+ Transport Through Intermediate Phase in High-Content Inorganic Composite Electrolytes” decodes the hidden chemistry that lets lithium sprint across solid/liquid boundaries.
The Secret Sauce: Acidic Interfaces
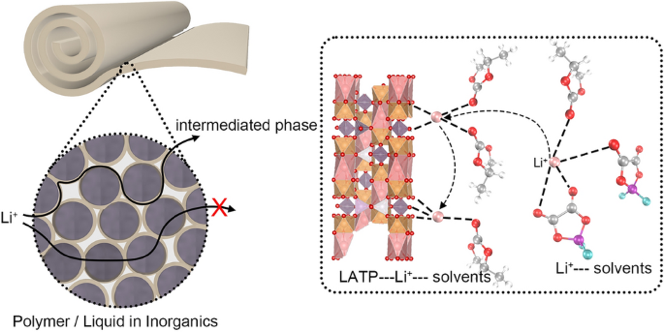
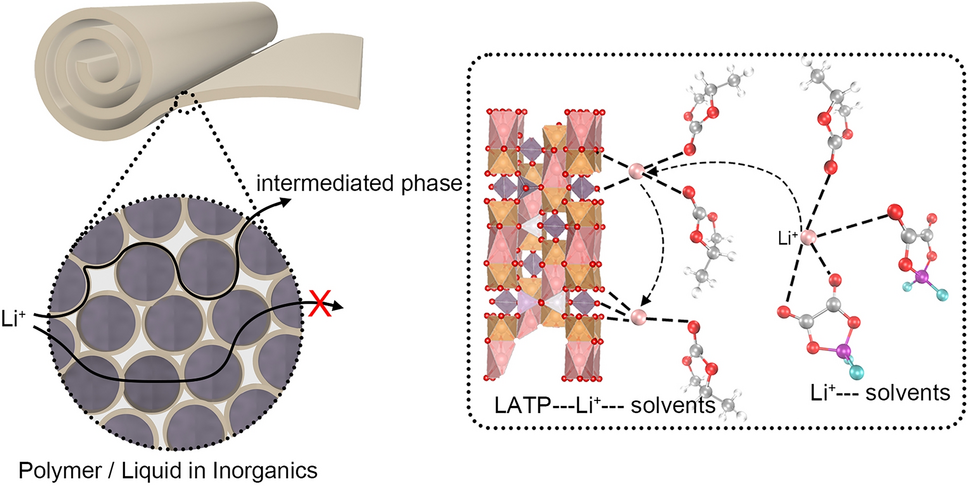
- Selective Anion Anchoring: Acidic LATP surfaces act as Lewis-acid traps for DFOB- anions, loosening Li+ solvation cages and jacking the Li+ transference number from 0.31 → 0.53.
- Size Matters: Shrinking LATP particles to 200–300 nm boosts specific surface area and pushes ionic conductivity to 51 mS cm-1 at room temperature.
- Dual-Phase Highways: An “intermediate phase” bridges ceramic and liquid domains, creating 3-D conduction networks that outpace single-phase polymers.
Performance that Speaks Louder than Theory
- 6000 h non-stop cycling in Li||Li symmetric cells at 0.1 mA cm-2 —no short-circuits.
- 5 % capacity retention after 200 cycles in a 5 V-class LNMO||Li full cell at 0.5 C.
- Pouch-cell demo drives LED arrays and mini-motors, proving scalability beyond coin cells.
Design Rules for Tomorrow's Electrolytes
- Surface Engineering > Bulk Chemistry: Acidic surface sites are the true catalysts; neutral or basic variants lag by 30 %.
- Active Fillers Win: Ion-conductive LATP beats inert alumina, cutting activation energy and sustaining high-rate capability (155 mAh g-1 at 0.1 C vs. 82 mAh g-1).
- SEI Self-Defense: LATP-induced decomposition forms a LiF-rich interphase that stops further Ti4+ reduction—self-limiting protection without extra coatings.
Future Outlook
- Nano-Architected Interfaces: Next-gen electrolytes will leverage tunable surface acidity and hierarchical porosity to push conductivities beyond 1 mS cm-1.
- High-Loading Cathodes: 14 mg cm-2LNMO cathodes already retain 142 mAh g-1—roadmap to >300 Wh kg-1 pouch cells.
- Universal Design Toolkit: The acid-base descriptor framework can be ported to sulfides, chlorides, and beyond, fast-tracking the commercial leap from lab to EV.
Stay tuned as the Yu–Huang team turns interfacial chemistry into the next performance revolution for lithium-metal batteries.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in