Escherichia coli can get (mechanically) stressed out too
Published in Microbiology
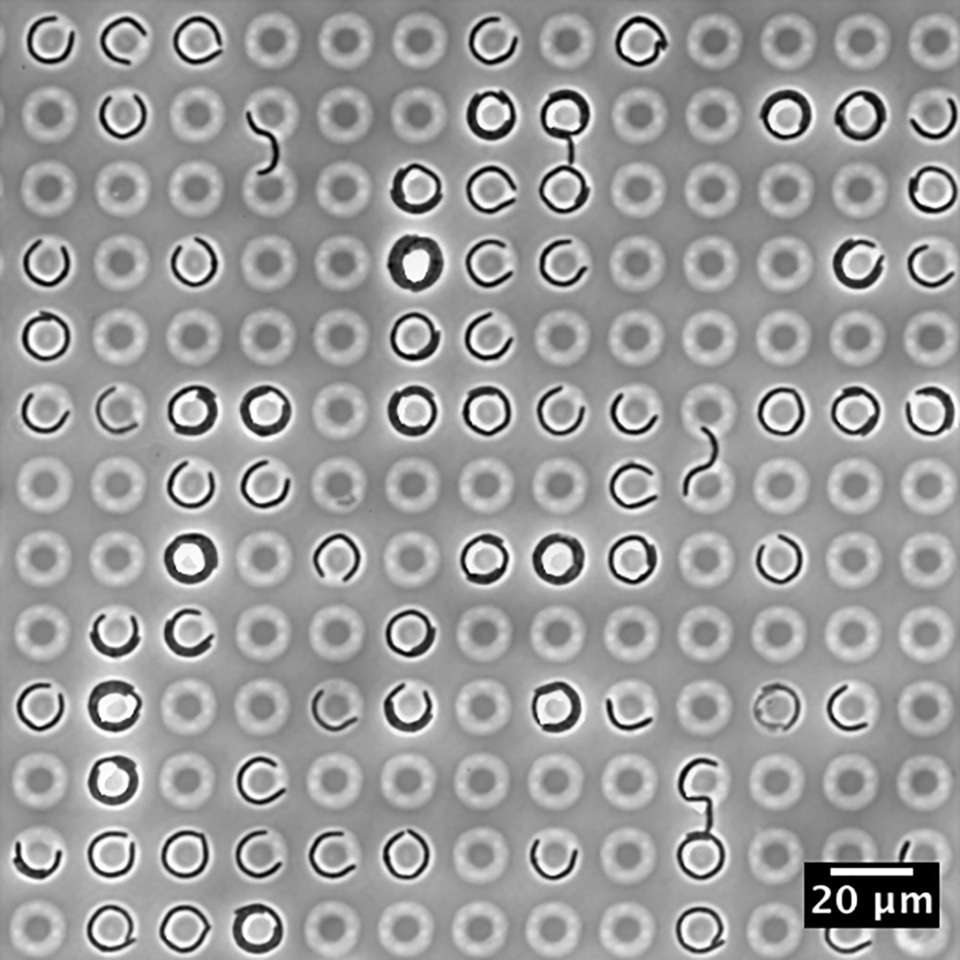
“To be brutally honest, few people care that bacteria have different shapes. Which is a shame, because the bacteria seem to care very much,” wrote Kevin Young in his 2006 review1. It seems that the field has matured quite a bit since then: microfluidic chambers were used to probe cellular morphology and motility in the years shortly after2,3; MreB, a prokaryotic actin homologue crucial to cell wall synthesis, was discovered to not be a cell-spanning helix but rather individual filaments in 20114-6; and recent work has dug deeper into different families of proteins responsible for cell wall synthesis, although several basic questions have remain unanswered (e.g., how does a cell maintain a constant diameter?). From a physics standpoint, theoretical work has suggested that mechanical stresses may influence the dynamics of cell wall growth7. This has led to an experimental study probing the influence of mechanical stresses on bacterial growth8, as we discuss below.
With the advent of microfluidics, (mechanically) stressing out bacteria has never been easier. In prior experiments, Ariel Amir et al. grew filamentous Escherichia coli and Bacillus subtilis cells in a mother machine and bent the cells with flow. Upon extinguishing the flow, the cells were still bent in the direction of the flow, suggesting that more wall growth had occurred along the edge facing the flow (where the cell wall was more stretched). Perhaps even more intriguingly, the cells straightened upon further growth, an observation that was left unresolved in that paper.
What could be responsible for this straightening? The two prevalent ideas in the field other than mechanical stress sensing has been (1) that a large processivity—the mean number of subunits incorporated into a glycan strand from initiation to termination of the elongation step—provides a built-in mechanism for straightening9 and (2) that proteins like MreB may localize to regions of negative Gaussian curvature and bias wall growth there10. We discuss why these two mechanisms likely do not paint the full picture in our paper. Predictably, we then asked whether it could be mechanical stresses that could explain both the biased growth when a cell is bent and straightening when it is free. Led by Lars Renner at the Leibniz Institute of Polymer Research and the Max Bergmann Center of Biomaterials, we devised a protocol for applying uniform bending forces in a high-throughput fashion similar to previous work by Douglas Weibel2. We then modeled the mechanical stresses experienced by the cell in this protocol.
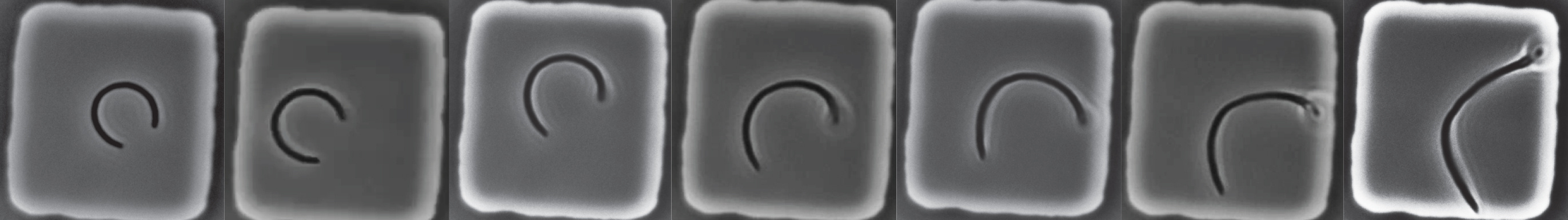
The protocol itself is simple and resembles footbinding of E. coli cells: filamentous cells were grown in tight, toroidal microchambers so as to become bent inside them, and the cells are released upon sufficient growth. We then tracked how fast they straightened. (We think of this procedure as footbinding because the cells are plastically deformed after being confined.)
Alongside these experiments, and with help from Jayson Paulose, we worked to theoretically model what happens if cell wall growth were coupled to mechanical stresses. We hypothesized that differential growth—where more or less wall material is added—depended on variations in the mechanical stresses, while the overall growth rate could be determined by other factors11 like metabolism. Our model not only predicted that the mechanical stresses qualitatively agreed with straightening, with larger stresses where the cell wall grows more, but also that single cells could straighten exponentially with a rate twice as fast as the growth rate. In other words, for every doubling of the cell length, the midline curvature would approximately be divided by four. This prediction, satisfyingly, was consistent with the empirical straightening rate.
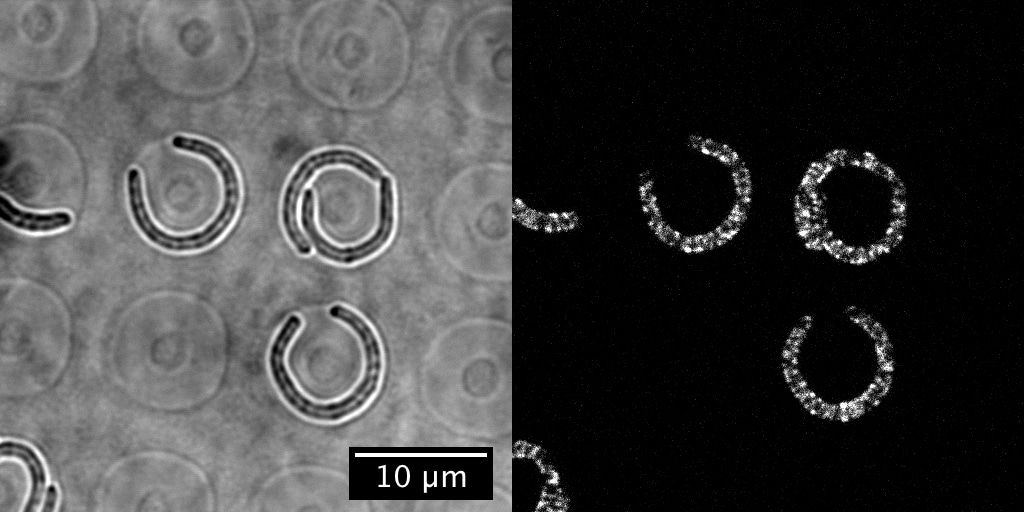
Can preferential MreB localization—as has been observed in cells with submicron-scale indentations10—account for the observed straightening or sense mechanical stress? By collaborating with Sven van Teeffelen, whose lab at Institut Pasteur has expertise in tracking MreB, and his PhD student, Gizem Özbaykal, we found that MreB localizes to the inner edge of bent cells regardless of confinement status (so, it would not be able to explain the snapback in previous flow experiments) and, interestingly, that the concentration of MreB at the inner edge also appears insufficient to explain straightening. This suggests that other molecular players are ultimately responsible for straightening.
Current work is now focused on more perturbations, which could validate the model further, as well as the possible molecular mechanisms that could detect mechanical stresses. Such mechanisms could have broad implications for shape adaptation in complex environments and normal cell growth (e.g. septum formation and wall thickness regulation).
The paper in Nature Microbiology is here: http://go.nature.com/2tME15K
1. Young, K. D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 70, 660–703 (2006).
2. Takeuchi, S., DiLuzio, W. R., Weibel, D. B. & Whitesides, G. M. Controlling the shape of filamentous cells of Escherichia coli. Nano Lett. 5, 1819–1823 (2005).
3. Mannik, J., Driessen, R., Galajda, P., Keymer, J. E. & Dekker, C. Bacterial growth and motility in sub-micron constrictions. Proc. Natl Acad. Sci. USA 106, 14861–14866 (2009).
4. Garner, E. C. et al. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science 333, 222–225 (2011).
5. Domínguez-Escobar, J. et al. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science 333, 225–228 (2011).
6. van Teeffelen, S. et al. The bacterial actin MreB rotates, and rotation depends on cell-wall assembly. Proc. Natl Acad. Sci. USA 108, 15822–15827 (2011).
7. Amir, A. & Nelson, D. R. Dislocation-mediated growth of bacterial cell walls. Proc. Natl Acad. Sci. USA 109, 9833–9838 (2012).
8. Amir, A., Babaeipour, F., McIntosh, D. B., Nelson, D. R. & Jun, S. Bending forces plastically deform growing bacterial cell walls. Proc. Natl Acad. Sci. USA 111, 5778–5783 (2014).
9. Sliusarenko, O., Cabeen, M. T., Wolgemuth, C. W., Jacobs-Wagner, C. & Emonet, T. Processivity of peptidoglycan synthesis provides a built-in mechanism for the robustness of straight-rod cell morphology. Proc. Natl Acad. Sci. USA 107, 10086–10091 (2010).
10. Ursell, T. S. et al. Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc. Natl Acad. Sci. USA 111, 1025–1034 (2014).
11. Rojas, E., Theriot, J. A. & Huang, K. C. Response of Escherichia coli growth rate to osmotic shock. Proc. Natl Acad. Sci. USA 111, 7807–7812 (2014).
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in