Establishing a link between G9a activity and pluripotency in colorectal cancer stem cells
Whenever I am giving lectures, I like to compare the organization of cellular heterogeneity within a tumor to the structure of an army, in which a small group of powerful individuals at the top of the hierarchy (i.e. the Generals) will dictate the progression of the troops.
Published in Cancer

Like
Be the first to like this
The Generals inside of a tumor mass are represented by cancer stem cells; a rare subset of self-renewing entities with tumor-initiating capacity, notoriously famous for evading chemo and radiotherapy. What is quite baffling about tumor heterogeneity is that two cells with identical genetic determinants can have completely distinct tumorigenic capacities. While a cancer stem cell can initiate and sustain the growth of an entire tumor, its neighbor just can’t achieve such a goal. According to recent literature on the topic, the key resides in the unique configuration of the epigenome of cancer stem cells1.
We had a particular interest for the histone methyltransferase G9a since its role was reported in the maintenance of leukemia stem cells2. Recently, G9a attracted much attention from the cancer research community with studies highlighting its pro-oncogenic function in melanoma, breast, and bladder cancers3-5.
Through our work, we established that colorectal tumors expressing highest levels of G9a mRNA generally showed a shorter disease-free survival rate, indicative of therapy failure and cancer reoccurrence. Then, looking at the transcriptome of these G9a-overexpressing patients, we found a higher degree of similarity with the transcriptional signature of pluripotent stem cells, compared to low G9a-expressing colon tumors. Such a correlation aligned with pioneer articles relating pluripotency to the concept of cancer stem cells6,7. I had the opportunity to use transformed human pluripotent cells in the past to model neoplastic stemness in culture8. When I established my own lab, my postdoc mentor Mick Bhatia had been very supportive and let me bring a transformed variant of human pluripotent cells, so I could use it to study epigenetic phenomenon governing cancer stem cell identity. Using well-documented G9a inhibitors, we were able to draw parallels between H3K9me2 deposition and transcriptional responses in colon cancer cell lines and transformed pluripotent cells. These findings mainly pointed toward a downregulation of pluripotency gene networks, self-renewal and the onset of differentiation programs. On a functional aspect, it was crucial to test whether G9a inhibition has an impact on the tumor-initiating capacity of cancer stem cells residing within human primary colorectal tumor samples. This recent paper in Oncogene was an opportunity to describe a serial organoid formation protocol that we developed in our lab, which “mimics” key aspects of the gold standard in vivo serial transplantation assay in a culture dish. Thus, we were able to demonstrate that G9a inhibition can significantly impair the tumor-initiating capacity of patient-derived colorectal tumor samples. Moreover, multi-omics data from colorectal cancer stem cell models showed that key pathways, such as Wnt and epithelium-to-mesenchyme transition (EMT) are controlled by H3K9me2 deposition, potentially explaining our observations from serial organoid plating experiments.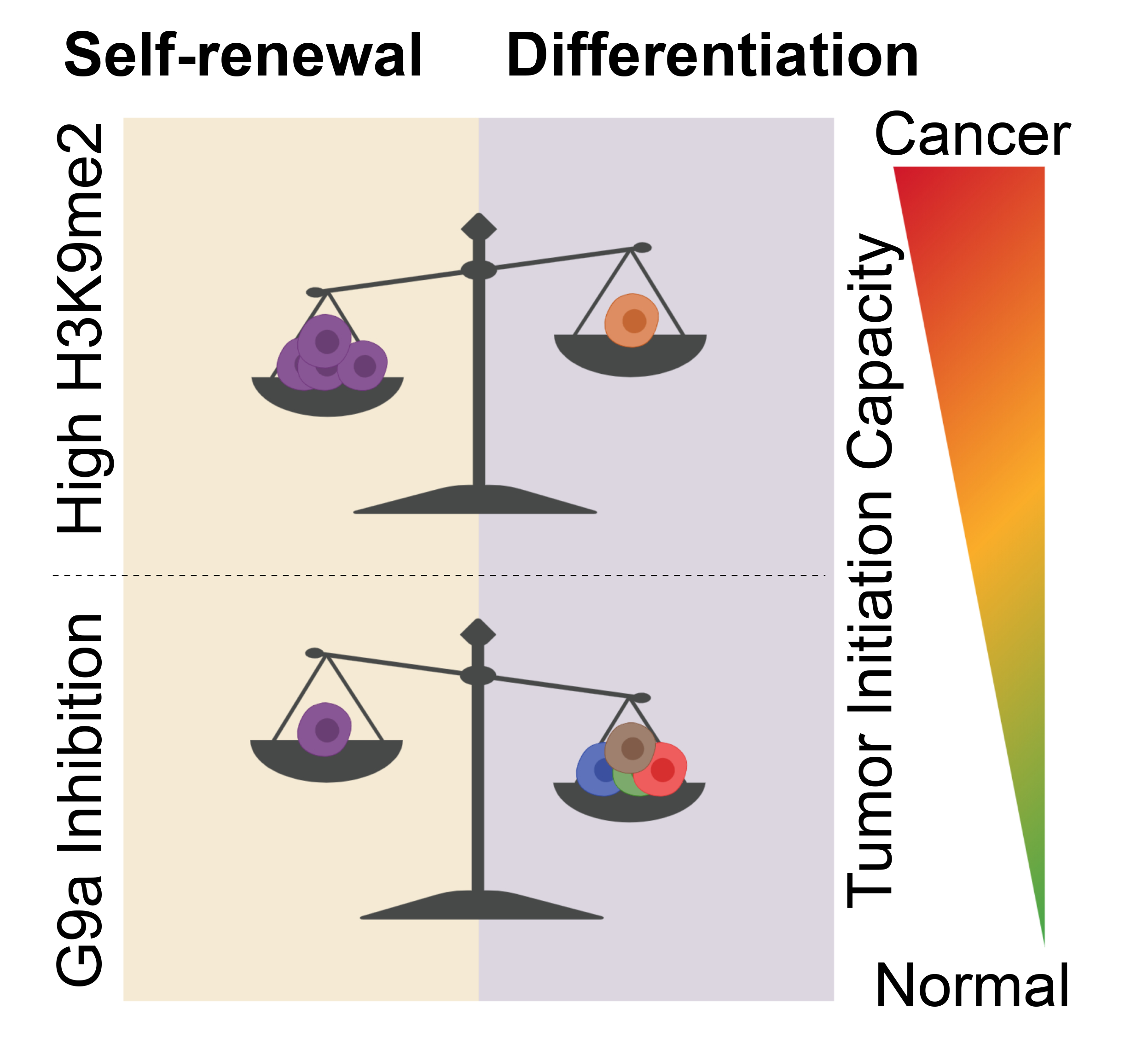

The take-home message from our work is that G9a inhibition represents an appealing strategy to block cancer stem cell activity. Several small molecule inhibitors were developed over the past fifteen years, including the ones we used in our study. Unfortunately, and to the best of my knowledge, none of these have yet made it to clinical phases. Additional work will be needed to eventually develop safe and potent tools targeting G9a in human patients.
- Wainwright, E. N. & Scaffidi, P. Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity. Trends Cancer3, 372-386, doi:10.1016/j.trecan.2017.04.004 (2017).
- Lehnertz, B.et al.The methyltransferase G9a regulates HoxA9-dependent transcription in AML. Genes Dev28, 317-327, doi:10.1101/gad.236794.113 (2014).
- Kato, S.et al.Gain-of-function genetic alterations of G9a drive oncogenesis. Cancer Discov, doi:10.1158/2159-8290.CD-19-0532 (2020).
- Mabe, N. W.et al.G9a Promotes Breast Cancer Recurrence through Repression of a Pro-inflammatory Program. Cell Rep33, 108341, doi:10.1016/j.celrep.2020.108341 (2020).
- Segovia, C.et al.Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat Med25, 1073-1081, doi:10.1038/s41591-019-0499-y (2019).
- Ben-Porath, I.et al.An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet40, 499-507, doi:10.1038/ng.127 (2008).
- Malta, T. M.et al.Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell173, 338-354.e315, doi:10.1016/j.cell.2018.03.034 (2018).
- Benoit, Y. D.et al.Sam68 Allows Selective Targeting of Human Cancer Stem Cells. Cell Chem Biol24, 833-844.e839, doi:10.1016/j.chembiol.2017.05.026 (2017).
Follow the Topic
Cancer Biology
Life Sciences > Biological Sciences > Cancer Biology
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in