Exploring and Targeting New Mechanisms of EGFR Inhibitor Resistance in Lung Cancer
Published in Cancer and Pharmacy & Pharmacology

Lung cancer, particularly non-small cell lung cancer (NSCLC), remains one of the leading causes of cancer-related deaths worldwide. Despite advancements in targeted therapies like epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), drug resistance often emerges, complicating treatment and leading to relapse. Our latest research uncovers crucial insights into the mechanisms behind drug resistance in EGFR-mutant lung cancer, offering new strategies to prevent or reverse resistance.
Understanding Drug Resistance
Lung cancer, especially among non-smokers and younger populations, is becoming more prevalent, creating new challenges in both understanding the disease and developing effective therapies. While treatments like EGFR TKIs, including osimertinib, have made significant strides in managing EGFR-mutant NSCLC, many patients eventually develop resistance. Some resistance mechanisms are known but often involve complex, multifactorial processes. In our study, we identified a critical form of resistance in EGFR-mutant tumors, which provides a more complete understanding of why therapies that initially show promise lose their effectiveness over time.
The Tumor Microenvironment and Its Role in Resistance
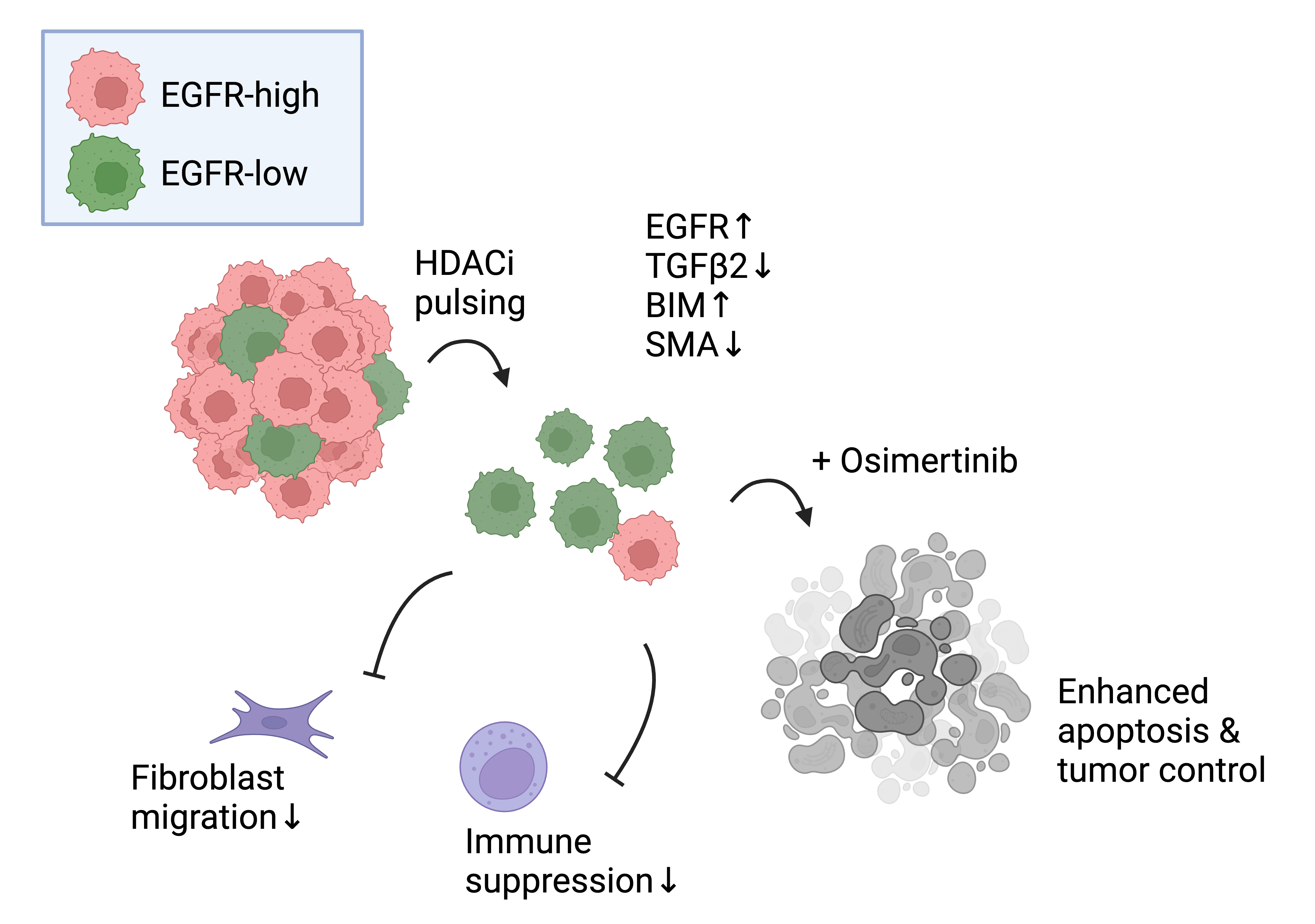
Drug resistance in cancer is not only about genetic mutations but also about how the tumor environment adapts to ongoing treatment. One of the key findings from our study was the role of the tumor microenvironment in supporting drug resistance. Specifically, we observed that EGFR-mutant tumors harbor clones of cancer cells that express low levels of both wild-type and mutant EGFR proteins. These EGFR-low cells are more tolerant to EGFR inhibitors and exhibit a more invasive, epithelial-to-mesenchymal-like phenotype compared to their EGFR-high counterparts.
These drug-tolerant cells also have a unique ability to influence the tumor microenvironment. They secrete Transforming Growth Factor Beta (TGFβ) family cytokines, which recruit cancer-associated fibroblasts and suppress the immune response, further promoting a drug-tolerant environment. This microenvironmental shift enhances cancer cells' ability to evade treatment and fuels tumor relapse. This insight is critical for understanding the early stages of drug resistance formation, particularly in the minimal residual disease (MRD) state, which occurs after initial treatment and can later lead to therapy failure.
.png)
The Significance of Intratumoral Heterogeneity
Intratumor heterogeneity—the diversity of cell populations within a tumor—has long been recognized as a major driver of therapy resistance. Our research provides further evidence that this heterogeneity is not only genetic but also functional. In our study, we demonstrated that EGFR-mutant NSCLC tumors exhibit pre-existing intratumoral heterogeneity in EGFR expression. This variation leads to distinct cell populations with low EGFR expression, which are more resilient to EGFR inhibitors. These findings underscore the complexity of drug resistance and the importance of understanding the different cellular populations within a tumor.
Our study builds on the understanding of how both genetic mutations and non-genetic factors, such as protein expression, contribute to tumor heterogeneity. Tumor cells with low mutant EGFR expression can have a significant impact on treatment outcomes, particularly in the early stages of drug resistance, before genetic mutations emerge. The role of epigenetic mechanisms in regulating gene expression and modifying the tumor microenvironment adds another layer of complexity to the resistance puzzle.
The Epigenetic Pathway: Targeting Resistance at the Molecular Level
Perhaps the most exciting aspect of our research lies in the discovery of epigenetic changes that could reverse or prevent drug resistance. Our study reveals that the EGFR-low cells exhibit epigenetic modifications that allow them to survive and evade therapy. By pharmacologically inducing EGFR expression in these resistant cells using epigenetic inhibitors, we were able to sensitize the cells to EGFR inhibitors, offering a promising strategy for overcoming resistance.
This finding highlights the potential of epigenetic therapies to restore the effectiveness of EGFR inhibitors. Epigenetic drugs work by modifying the expression of genes without altering the underlying DNA sequence, and they can be used to reverse the adaptations that cancer cells have made to resist treatment. When combined with traditional therapies like EGFR TKIs, these drugs could provide a powerful new way to combat resistance and improve patient outcomes.
The combination of epigenetic therapies with existing cancer treatments offers a promising approach to overcoming resistance. By targeting the tumor’s genetic and microenvironmental resistance mechanisms simultaneously, combination therapies may be more effective than single-agent treatments. In our study, we found that combining epigenetic drugs with EGFR inhibitors could prevent the development of resistance, providing a more durable and effective treatment strategy.
Expanding Beyond Lung Cancer: Implications for Other Cancers
While our research focused primarily on lung cancer, the insights we’ve uncovered have broader implications for drug resistance in other cancers. Drug resistance is not unique to lung cancer; it is a common challenge in many cancer types, including breast, colon, and pancreatic cancer. The mechanisms we’ve identified in EGFR-mutant lung cancer may apply to other cancers as well, potentially leading to new therapeutic strategies for overcoming resistance across different tumor types.
Towards Personalized Cancer Therapies
The ultimate goal of our research is to translate these discoveries into more personalized treatments for cancer patients. By identifying the specific mechanisms that contribute to drug resistance, we can tailor treatments to target these mechanisms directly, offering more effective and individualized therapies. Epigenetic drugs (more specifically histone deacetylase inhibitors, HDACi), when used in combination with EGFR TKIs, could provide a way to restore the effectiveness of existing therapies, giving patients a better chance at survival.
While this research is still in its early stages, the potential for clinical applications is vast. As we continue to study these mechanisms and test new treatments, we are hopeful that these findings will lead to improved therapies.
Looking Ahead: Hope on the Horizon
Overcoming drug resistance in cancer is a significant challenge, but our study provides hope for the future. By targeting the underlying causes of resistance, we can develop therapies that are more effective and less prone to failure. As clinical trials begin to explore the use of epigenetic drugs in combination with EGFR TKIs, we are optimistic that these breakthroughs will lead to better outcomes for cancer patients.
This research work was conducted as a collaboration between multiple institutions, including University of Helsinki, Dana-Farber Cancer Institute & Harvard Medical School.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in