Exploring Prolonged Drug Action: The Role of Epichaperome Agents
Published in Cancer and Pharmacy & Pharmacology
Exploring Prolonged Drug Action: The Role of Epichaperome Agents
Have you ever wondered why some drugs seem to work longer and more effectively than others? The answer might lie in a fascinating cellular structure known as the epichaperome. Researchers at Memorial Sloan Kettering Cancer Center have been delving into this intricate world, and their findings could change the way we think about drug treatments.
The Power of Drug Residence Time
In the realm of drug development, one crucial factor often determines a drug's effectiveness: residence time. It's the amount of time a drug lingers at its target site. Drugs with a long residence time tend to be more efficacious in treating diseases. But understanding why and how some drugs have this extended residence time has been a challenge, especially for non-covalent agents.
Enter the Epichaperome Agents
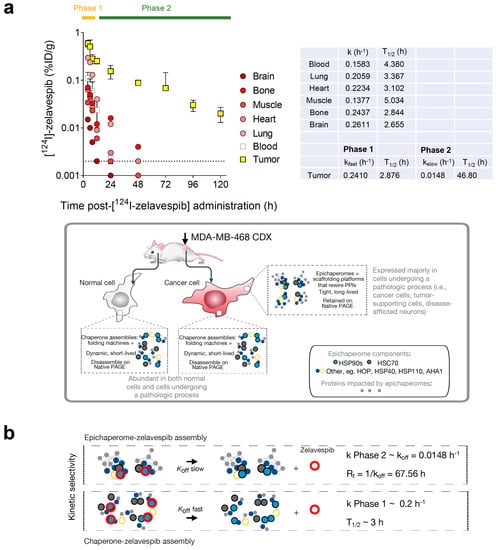
Enter epichaperome agents like zelavespib and icapamespib. These compounds have intrigued researchers for their ability to remain bound to their target for days, even after rapid clearance from plasma, minimal presence in non-diseased tissues, and fast metabolism. It's like they've cracked the code to sustained drug action.
The Epichaperome: A Molecular Hub
So, what exactly is the epichaperome? It's a complex molecular structure composed of tightly bound chaperones, co-chaperones, and other factors. Unlike chaperones, which are ubiquitous in cells, epichaperomes are primarily found in diseased cells and tissues. They play a unique role in rewiring protein-protein interactions at a proteome-wide level.
Unraveling the Mechanism
To get to the bottom of this mystery, the researchers conducted a comprehensive study. They monitored drug concentration, target occupancy, and epichaperome assemblies in a live mouse model. What they discovered was truly enlightening.
The Trapping Effect
One key finding was the trapping effect. Initially, epichaperome agents become trapped upon binding. This mechanism leads to the disassembly of epichaperomes, all without altering the expression level of epichaperome constituents. It's a process that seems to be a major contributor to the extended on-target residence time observed with these agents in clinical settings.
Implications for Drug Development
These findings have profound implications for drug development. They provide a deeper understanding of why some drugs outperform others and offer new avenues for enhancing drug efficacy. By targeting the trapping effect and manipulating drug residence time, researchers may unlock new treatment strategies for various diseases.
Conclusion
The world of drug development is constantly evolving, and the discovery of epichaperome agents' unique mechanism adds another layer to our understanding. With more research in this area, we could see the development of highly effective drugs that revolutionize disease treatment.
Stay tuned for further developments in this exciting field. The secret behind prolonged drug action is slowly being unraveled, and it promises to change the landscape of medicine as we know it.
References
Sharma S, Joshi S, Kalidindi T, Digwal CS, Panchal P, Lee S-G, Zanzonico P, Pillarsetty N, Chiosis G. Unraveling the Mechanism of Epichaperome Modulation by Zelavespib: Biochemical Insights on Target Occupancy and Extended Residence Time at the Site of Action. Biomedicines. 2023; 11(10):2599. https://doi.org/10.3390/biomedicines11102599
Bolaender A, Zatorska D, He H, Joshi S, Sharma S, Digwal CS, Patel HJ, Sun W, Imber BS, Ochiana SO, Patel MR, Shrestha L, Shah SK, Wang S, Karimov R, Tao H, Patel PD, Martin AR, Yan P, Panchal P, Almodovar J, Corben A, Rimner A, Ginsberg SD, Lyashchenko S, Burnazi E, Ku A, Kalidindi T, Lee SG, Grkovski M, Beattie BJ, Zanzonico P, Lewis JS, Larson S, Rodina A, Pillarsetty N, Tabar V, Dunphy MP, Taldone T, Shimizu F, Chiosis G. Chemical tools for epichaperome-mediated interactome dysfunctions of the central nervous system. Nat Commun. 2021 Aug 3;12(1):4669. doi: 10.1038/s41467-021-24821-2.
Pillarsetty N, Jhaveri K, Taldone T, Caldas-Lopes E, Punzalan B, Joshi S, Bolaender A, Uddin MM, Rodina A, Yan P, Ku A, Ku T, Shah SK, Lyashchenko S, Burnazi E, Wang T, Lecomte N, Janjigian Y, Younes A, Batlevi CW, Guzman ML, Roboz GJ, Koziorowski J, Zanzonico P, Alpaugh ML, Corben A, Modi S, Norton L, Larson SM, Lewis JS, Chiosis G, Gerecitano JF, Dunphy MPS. Paradigms for Precision Medicine in Epichaperome Cancer Therapy. Cancer Cell. 2019 Nov 11;36(5):559-573.e7. doi: 10.1016/j.ccell.2019.09.007.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in