First clinical proof-of-concept of CAR T-cell combination therapy: The phase 2, single-arm ZUMA-14 trial of axicabtagene ciloleucel in combination with rituximab for refractory large B-cell lymphoma
Published in Cancer, General & Internal Medicine, and Pharmacy & Pharmacology
Rationale for the ZUMA-14 Clinical Trial
Large B-cell lymphoma (LBCL) is a fast-growing blood cancer that forms when B cells, a type of white blood cell, begin to grow abnormally. Chimeric antigen receptor (CAR) T-cell therapy has dramatically improved outcomes for people with LBCL, including increasing their survival.1 CAR T-cell therapy is an anti-cancer treatment in which a person’s T cells are modified in a laboratory to better recognize and kill cancer cells, then infused back into the person. Axicabtagene ciloleucel (axi-cel) is a CAR T-cell therapy that targets a specific marker (referred to scientifically as an antigen), called CD19, on the surface of cancerous B cells. Axi-cel is approved by regulatory agencies in many countries to treat people with LBCL who have had at least 1 prior treatment for their cancer.2 Some people who get axi-cel will have their cancer come back after treatment, called relapse.3 This may be because the cancer cells lose the CD19 marker on their cell surface after treatment, or the cancer cells may not have had this marker before treatment. When researchers studied tumors from these people, they found that cancer cells that did not have the CD19 marker often had a different cell surface marker, called CD20.3
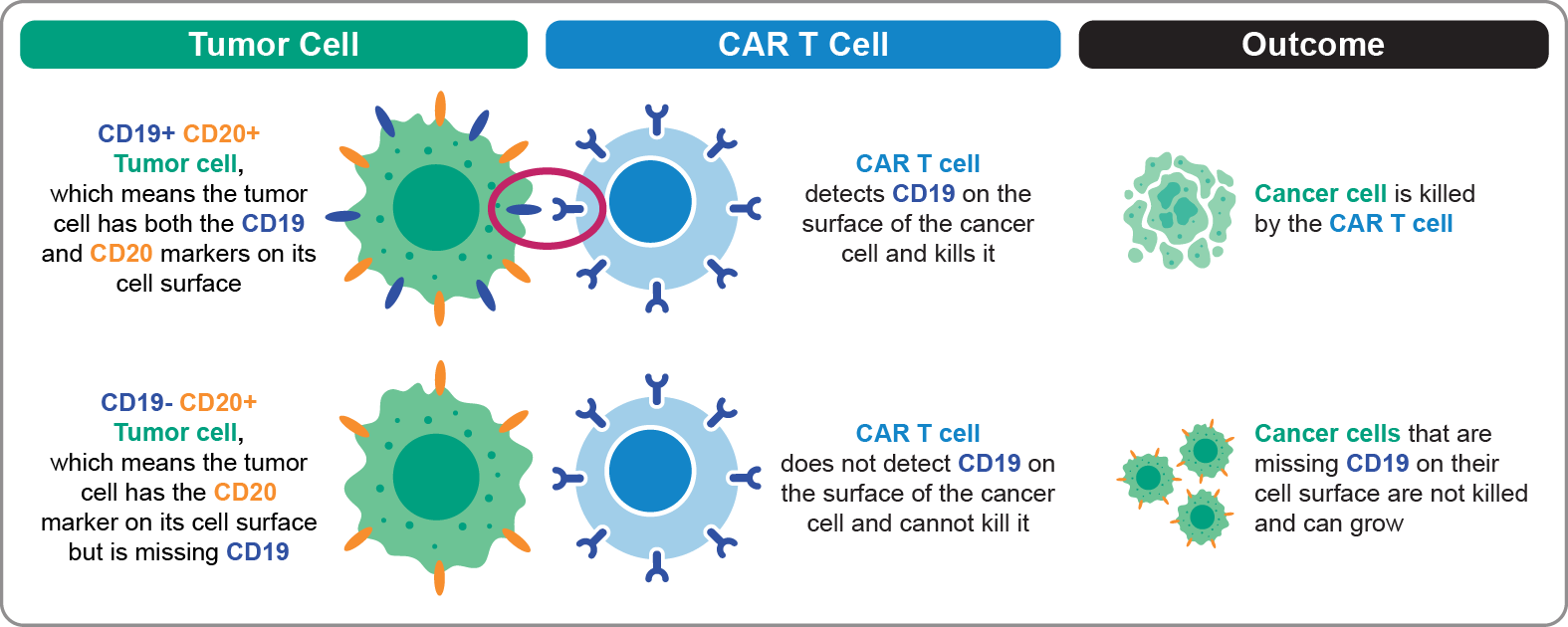
As shown in the top row of Figure 1 (below), a tumor cell that has both CD19 and CD20 markers on its cell surface is killed by the CAR T cell (axi-cel). This is because the CAR T cell detects CD19 on the surface of the cancer cell and kills it. However, a tumor cell that is missing CD19 and has only the CD20 marker on its cell surface will not be killed by CAR T cells directed at CD19, as shown in the bottom row of Figure 1. In this case, the cancer cells that have only the CD20 marker remain alive and can grow.
Figure 1. Single-Antigen–Targeting Approach: Anti-CD19 CAR T-Cell Therapy (Axi-Cel)
Treatments that target CD19 and CD20 markers, referred to as a dual-antigen–targeting approach, may better kill cancer than treatments that target only CD19. The ZUMA-14 clinical trial explored this idea.4 In ZUMA-14, we looked at how safe and well the combination of axi-cel and rituximab worked to treat people with refractory LBCL. Rituximab is a monoclonal antibody that binds to CD20 on the surface of B cells, like the cancerous cells in LBCL.
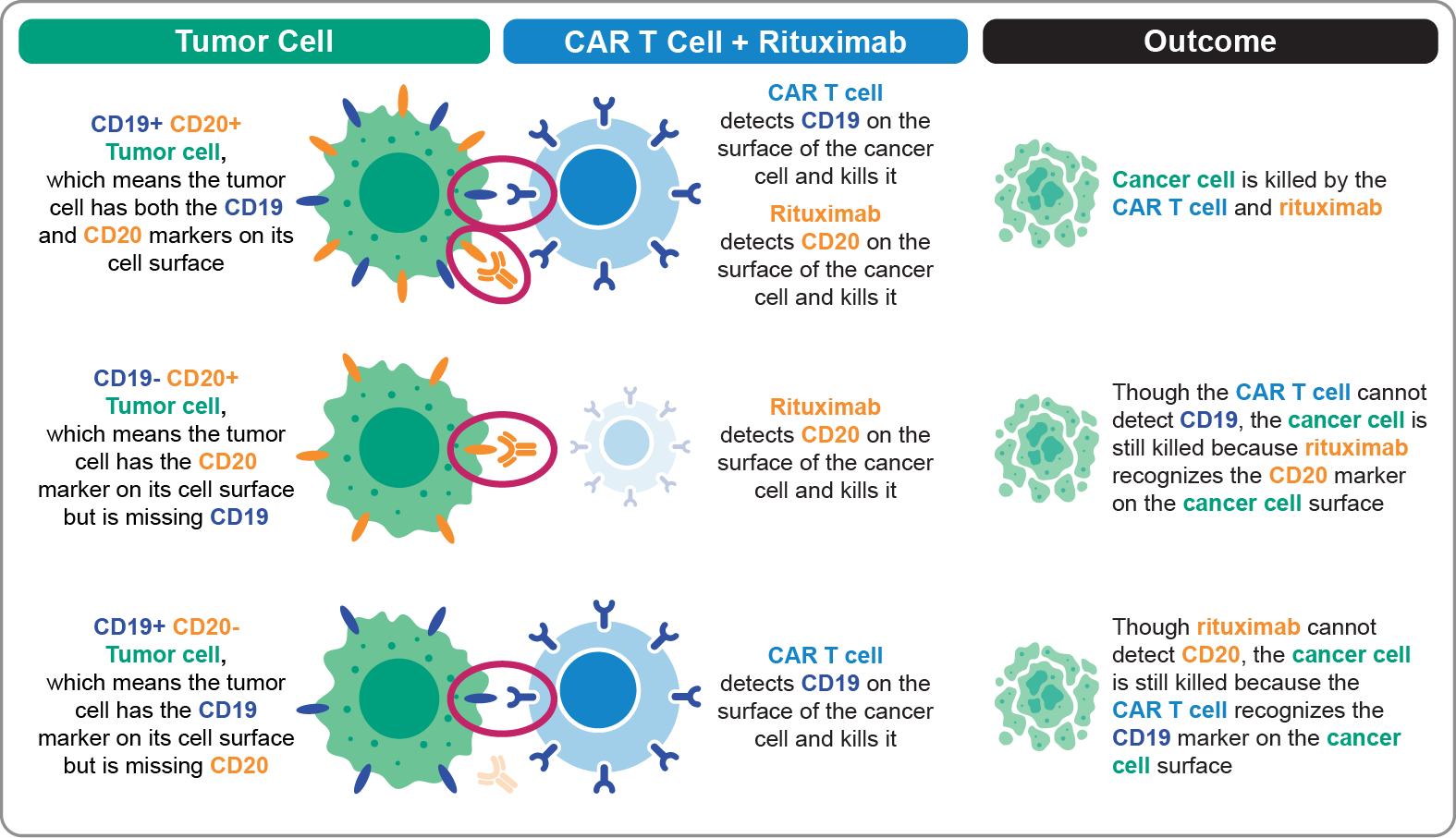
Our hypothesis was that a tumor cell with CD19 and CD20 markers on its cell surface can be killed by the CAR T cell (axi-cel) or by rituximab, as shown in the top row of Figure 2 (below). If a tumor cell is missing CD19 and only has the CD20 marker, it will not be killed by the CAR T cell, as shown in the middle row of Figure 2. Unlike in Figure 1, where these CD19-missing CD20-positive tumor cells can grow, the tumor cell is killed using a dual-targeting combination. This is because rituximab detects CD20 on the cancer cell’s surface and causes the cancer cell to die. Lastly, a tumor cell that is missing CD20 and only has the CD19 marker on its cell surface is killed by the CAR T cell, as shown in the bottom row of Figure 2. Altogether, a dual-antigen–targeting approach may help detect, target, and kill different types of cancer cells better than a single-antigen–targeting approach.
Figure 2. Dual-Antigen–Targeting Approach: Combination Therapy With Anti-CD19 CAR T-Cell Therapy (Axi-Cel) Plus Anti-CD20 Monoclonal Antibody (Rituximab)
In ZUMA-14, we looked at how well axi-cel and rituximab worked together by measuring several outcomes. We measured the depth and duration that cancer stayed undetectable for study participants, and how long participants stayed alive without their cancer coming back after treatment or dying for any reason. We looked at how safe axi-cel and rituximab was by measuring how many people had common side effects of CAR T-cell therapy, such as cytokine release syndrome (CRS), neurologic events, and infections. CRS is a strong immune reaction that causes the release of certain chemicals that result in symptoms such as fever, low blood pressure, low oxygen levels, or fatigue. Neurologic events are negative changes in a person’s nervous system. The grade, or severity, of side effects is assessed by a person’s doctor. Severe effects are those that are Grades 3, 4, or 5 on a 5-point scale.
Lastly, we compared data from people in ZUMA-14 with a group of people, or a cohort, in a different clinical trial, called ZUMA-1.5 People in ZUMA-1 cohort 1 got axi-cel for their LBCL. This let us see how well the dual-antigen–targeting approach (like in Figure 2) in ZUMA-14 compared with a single-antigen–targeting approach (like in Figure 1) in ZUMA-1. It is important to mention that people in ZUMA-14 were not perfectly matched with people in ZUMA-1 cohort 1. Although people in both studies had LBCL, their disease and number of prior treatments were different. This means that comparisons between the 2 studies should be made carefully without overinterpreting the findings.
Key Findings
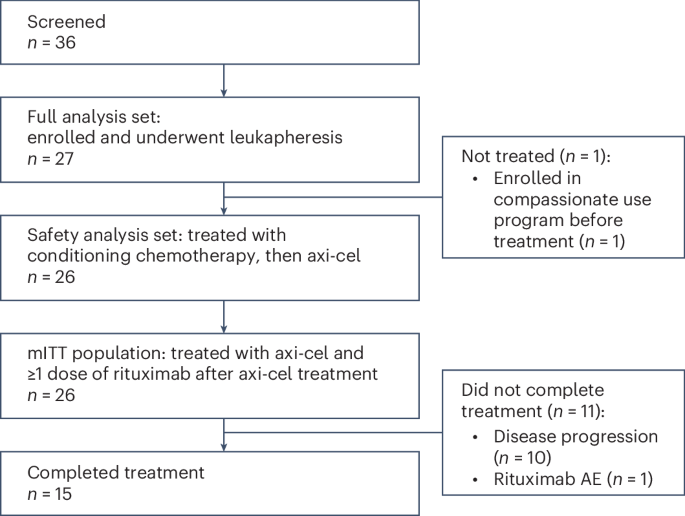
People were enrolled in ZUMA-14 between November 18, 2019, and November 6, 2020. Of 27 people who were enrolled, 26 got axi-cel and at least 1 dose of rituximab. People in the study were followed for a median (middle value) of 25.1 months.
Treatment with axi-cel and rituximab in ZUMA-14 appeared to work better and was generally safer for people with refractory LBCL compared with axi-cel alone (ZUMA-1 cohort 1). These outcomes are summarized in the Table below. At 24 months, 73% of people in ZUMA-14 had a complete response, or no signs of cancer, compared with 53% of people in ZUMA-1 cohort 1. The 24-month estimated duration of response (DOR) rate was 54.5% in ZUMA-14 and 39.8% in ZUMA-1 cohort 1. DOR refers to how long people who responded to treatment had their cancer stay small or undetectable after treatment. The estimated rates of survival at 24 months, including progression-free survival (PFS) and overall survival (OS), were also higher among people in ZUMA-14 compared with ZUMA-1 cohort 1. PFS refers to how long people lived after treatment without their cancer getting worse or dying from any cause. OS refers to how long people lived after treatment until they died, including deaths not from cancer. These outcomes show that more people in ZUMA-14 survived to 24 months after treatment versus people in ZUMA-1 cohort 1.
We observed fewer severe side effects among people who got the combination of axi-cel and rituximab in ZUMA-14 compared with people who got axi-cel in ZUMA-1 cohort 1 (Table). One reason for this may be the timing of when the clinical trials took place. ZUMA-14 started after ZUMA-1, so researchers may have had better understanding of these events and how to prevent and treat them early. No people in ZUMA-14 had severe CRS; 10 people (13%) in ZUMA-1 cohort 1 had CRS. Four people (15%) in ZUMA-14 had severe neurologic events compared with 22 people (29%) in ZUMA-1 cohort 1. The number of people who had severe infections was generally similar between the 2 studies, with 23% in ZUMA-14 and 26% in ZUMA-1 cohort 1. Lastly, adding rituximab in ZUMA-14 did not have a negative impact on how well the CAR T cells in the axi-cel product could multiply, or quickly grow in number, after detecting a tumor.
Table. Comparison of Outcomes Between People in ZUMA-14 and ZUMA-1 cohort 1
| Outcomes |
ZUMA-14 (N=26) |
ZUMA-1 cohort 1 (N=77) |
| Treatment strategy | Dual-antigen (CD19 and CD20) |
Single-antigen (CD19 only) |
| Efficacy: how well treatment worked at 24 months | ||
| Complete response rate, % | 73% | 53% |
| Duration of response rate, % | 54.5% | 39.8% |
| Progression-free survival rate, % | 48.2% | 32.8% |
| Overall survival rate, % | 72.9% | 45.5% |
| Safety: how safe treatment was | ||
| Grade ≥3 cytokine release syndrome, n (%) | 0 (0%) | 10 (13%) |
| Grade ≥3 neurologic events, n (%) | 4 (15%) | 22 (29%) |
| Grade ≥3 infections, n (%) | 6 (23%) | 20 (26%) |
Overall, ZUMA-14 showed that dual-antigen–targeting of CD19 and CD20 with axi-cel and rituximab, respectively, is a promising and generally safe strategy for people with refractory LBCL. People in ZUMA-14 had responses to treatment that were meaningful and durable, or long-lasting. Some people had severe side effects that researchers previously saw with these therapies. However, there were no unexpected safety events.
Future Directions
ZUMA-14 is a proof-of-concept for combining CAR T-cell therapy with another treatment. The trial allowed us to better understand how well and safe a dual-antigen–targeting approach is for people with LBCL who need more treatment for their cancer. Although the results from ZUMA-14 show the benefit of this treatment strategy, the findings need to be confirmed in another clinical study.
The findings from this study may also help guide development of future anti-cancer treatments. Rather than targeting 2 cancer cell markers with 2 separate therapies, like in this study, it may be preferred to have 1 treatment that is designed specifically to target 2 markers. Researchers are working to develop and test CAR T-cell products that target 2 cancer cell markers, like CD19 and CD20, within 1 anti-cancer treatment. As a dual-antigen–targeting treatment approach may limit the chances for tumor cells to escape detection and cell death compared with a single-antigen–targeting approach, these new therapies may help to further improve outcomes for people with LBCL who require more treatment.
References
- Neelapu, S. S. et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141, 2307-2315 (2023).
- YESCARTA® (axicabtagene ciloleucel) Prescribing information. Kite Pharma, Inc; 2025.
- Plaks, V. et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood 138, 1081-1085 (2021).
- Safety and Efficacy of Axicabtagene Ciloleucel in Combination With Rituximab in Participants With Refractory Large B-Cell Lymphoma (ZUMA-14). National Institutes of Health ClinicalTrialgs.gov. Published February 20, 2024. Accessed December 22, 2025. https://clinicaltrials.gov/study/NCT04002401.
- Study Evaluating the Safety and Efficacy of KTE-C19 in Adult Participants With Refractory Aggressive Non-Hodgkin Lymphoma (ZUMA-1). National Institutes of Health ClinicalTrialgs.gov. Published June 4, 2024. Accessed December 22, 2025. https://clinicaltrials.gov/study/NCT02348216.
Acknowledgments
We thank the participants who participated in the ZUMA-14 trial and their families, caregivers and friends, along with the trial investigators, coordinators, and healthcare staff at each study site. Medical writing support was provided by Ashley Skorusa, PhD, CMPP, of Nexus Global Group Science, funded by Kite, a Gilead Company. The ZUMA-14 trial was sponsored by Kite. P. Strati is supported by the Blood Cancer United Scholar in Clinical Research Award.
Follow the Topic
-
Nature Cancer

This journal aims to provide a unique forum through which the cancer community will learn about the latest, most significant cancer-related advances across the life, physical, applied and social sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in