Flexible tactile sensors based on gold nanoparticles-precipitated carbon nanotubes with low contact resistance and high sensitivity
Published in Healthcare & Nursing and Materials
This research introduces a flexible tactile sensor made from a 3D porous sponge structure coated with carbon nanotubes (CNTs) that are directly decorated with gold nanoparticles (AuNPs).
- CNTs are excellent conductors and mechanically flexible but suffer from high contact resistance where tubes touch.
- AuNPs can improve electron transfer at those contact points.
- When AuNPs are directly precipitated on CNT surfaces (not just mixed together), they form strong electrical bonds that lower contact resistance dramatically.
By integrating these materials within a porous polydimethylsiloxane (PDMS) sponge, a conductive network was formed in which the contact resistance among the AuNPs-precipitated CNTs varies significantly under applied pressure, thereby achieving high sensitivity even under high-pressure conditions.
This study presents a flexible tactile sensor that combines high sensitivity with a wide pressure detection range by integrating gold-nanoparticle-decorated carbon nanotubes (AuNP–CNTs) into a porous sponge structure made of PDMS. Tactile sensors are essential for wearable electronics, healthcare monitoring, and robotic applications, but most current designs face a trade-off between sensitivity and sensing range. To overcome this limitation, we developed a simple and scalable fabrication method that uses a “wet chemical precipitation” process to directly grow gold nanoparticles on the surface of carbon nanotubes. These AuNP–CNTs were then coated onto a porous PDMS sponge through a dipping and drying process, creating a three-dimensional conductive network that changes its resistance when compressed (Figure 1). The direct precipitation of AuNPs on CNTs significantly reduces contact resistance and enhances electrical conductivity compared with sensors that use CNTs alone or mixtures of CNTs and AuNPs. When pressure is applied, the number of contact points between AuNP–CNTs increases, resulting in a clear and repeatable current change that allows precise pressure detection.
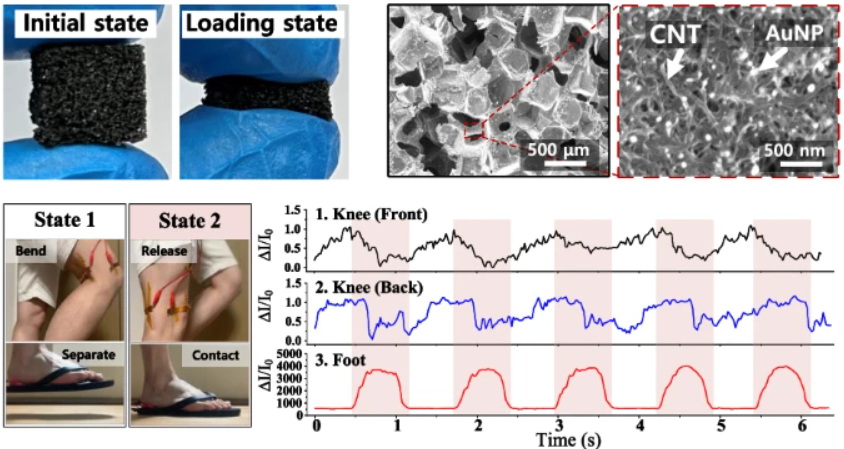
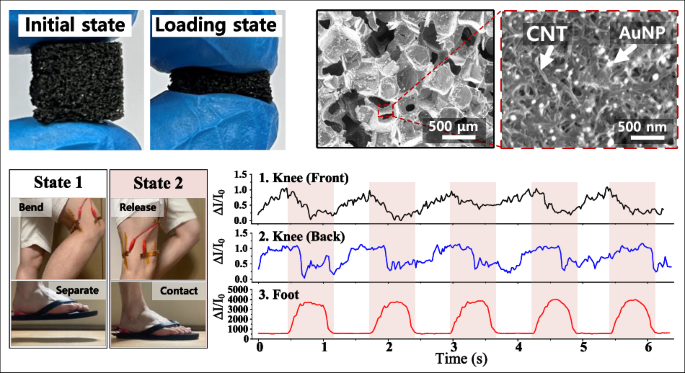
We demonstrated practical applications in wearable healthcare (Figure 2). When attached to a user’s wrist, the sensor detected clear arterial pulse waveforms with distinct peaks corresponding to percussion, tidal, and diastolic signals, enabling the measurement of heart rate and vascular stiffness. Attached to the neck, it successfully monitored swallowing motions with a current increase of about 300 %, suggesting potential use in diagnosing swallowing disorders. When placed on the cheek, it distinguished chewing intensity—showing roughly fourfold larger signals for hard food compared to soft food—indicating possible applications in jaw motion or temporomandibular disorder analysis. The sensor also captured large-scale movements such as walking; sensors attached to the knees and foot accurately differentiated between lifting and stepping actions during gait cycles. These results demonstrate that the same sensor can measure both subtle physiological signals and high mechanical pressures with stability and reproducibility.
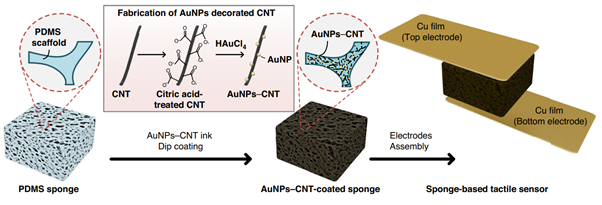
Figure 1. Schematic illustration of the fabrication process of AuNPs–CNTs-coated sponge-based tactile sensor. The tactile sensor consists of the AuNPs–CNTs-coated sponge and copper electrodes.
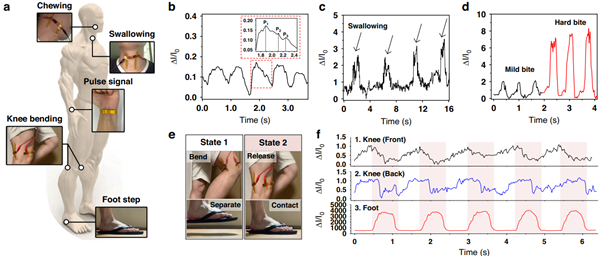
Figure 2. Demonstration of our tactile sensor for health-monitoring applications. a Schematic illustration and optical photographs of the tactile sensor for measuring healthcare signals (Chewing, swallowing, pulse signal, knee bending, and footstep). b Pulse signal of the user. The enlarged pulse waveform shows percussion (P1), tidal (P2), and diastolic (P3) peaks for diagnosing arterial disease. c Neck muscle movement. d Cheek muscle movement. e Photographs of the knee and foot when the user is walking. The walking process was divided into taking off (state 1) and stepping on (state 2). f Monitoring the signals of the tactile sensors attached to the front of the knee, the back of the knee, and the foot.
Publication: https://doi.org/10.1038/s41378-025-01056-5
Follow the Topic
-
Microsystems & Nanoengineering

This journal, with a target for a high-end journal for years to come, seeks to promote research on all aspects of microsystems and nanoengineering from fundamental to applied research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in