Formation Mechanisms of Biorelevant Lactaldehyde and Its Isomers in Interstellar Ice Analogs
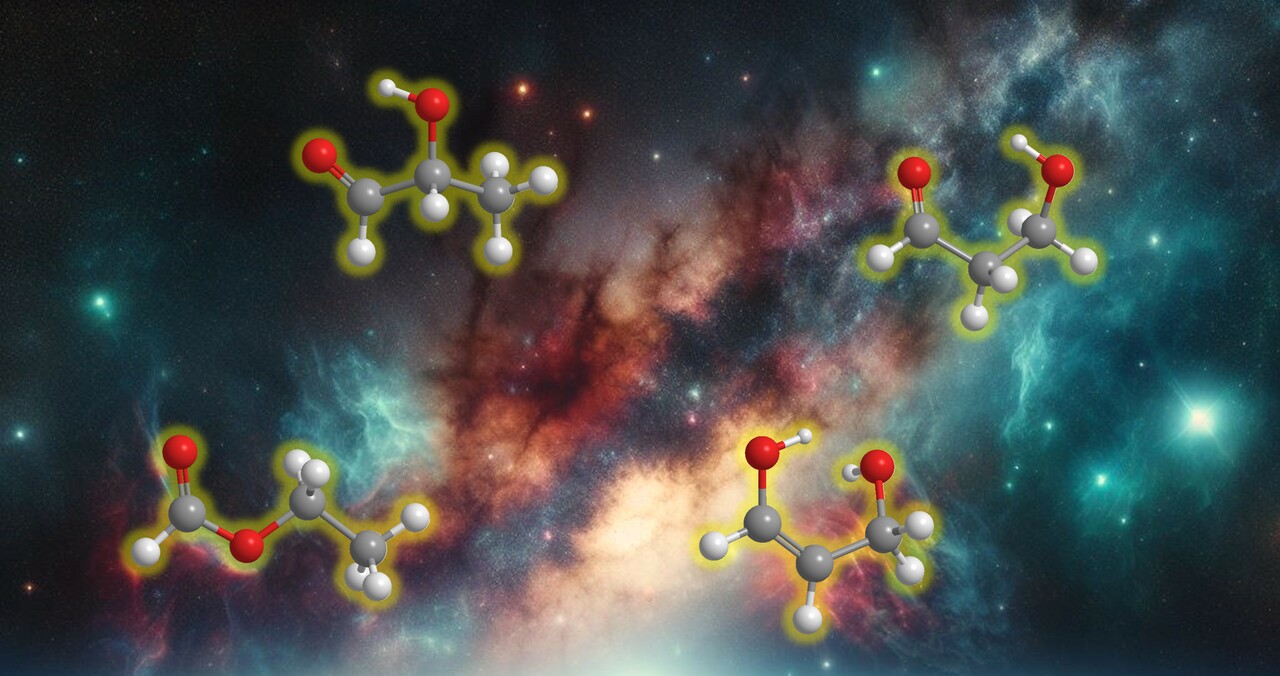
Over the last half-century, interstellar aldehydes (RCHO), with R being organic groups, have garnered significant attention due to their role as crucial intermediates in metabolic pathways and key precursors to biorelevant molecules critically linked to the molecular origins of life. For example, the simple aldehyde acetaldehyde (CH3CHO) is ubiquitous in the interstellar medium (ISM) and has been tentatively identified in interstellar ices. It can react with carbon dioxide (CO2) in interstellar environment to form the biorelevant pyruvic acid (CH3COCOOH), a key building block of metabolites and amino acids1. Among the more than 300 molecules identified in the ISM, 9 aldehydes were detected2. Furthermore, 16 aldehydes, including the simplest one, formaldehyde (H2CO), have been detected in carbonaceous chondrites3, indicating that aldehydes can form in astrophysical environments and survive the entrance of the parent bodies of the meteorites into the atmosphere of the early Earth4. Despite their significance, the interstellar formation mechanisms of complex, biologically relevant aldehydes have remained largely elusive.
Laboratory experiments were conducted to investigate the formation pathways of biorelevant lactaldehyde and its isomers in processed interstellar ice analogs composed of carbon monoxide (CO) and ethanol (CH3CH2OH) within an ultrahigh vacuum chamber (a few 10−11 Torr).The mixed ices were prepared by depositing a gas mixture of carbon monoxide and ethanol vapor onto a polished silver substrate, cooled to 5 K. The ices were irradiated with energetic electrons to simulate the secondary electrons generated by galactic cosmic rays (GCRs) in cold molecular clouds aged up to seven million years. After processing, the ices were heated to 320 K via a temperature-programmed desorption (TPD) process, mimicking the transition from a cold molecular cloud to a star-forming region. We developed a photoionization reflectron time-of-flight mass spectrometry (PI-ReToF-MS) technique to identify the reaction products during TPD isomer-specifically based on their desorption temperatures and calculated adiabatic ionization energies5. Utilizing isotopic substitution studies and tunable vacuum ultraviolet (VUV) PI-ReToF-MS, lactaldehyde (CH3CH(OH)CHO)and its isomers—3-hydroxypropanal (HOCH2CH2CHO), ethyl formate (CH3CH2OCHO), and 1,3-propenediol (HOCH2CHCHOH)—were identified in the gas phase6. These results suggest that lactaldehyde (CH3CH(OH)CHO)forms via barrierless recombination of formyl (HĊO) and 1-hydroxyethyl (CH3ĊHOH) radicals, revealing fundamental formation pathways for complex, biologically relevant aldehydes through non-equilibrium reactions under astrophysical conditions6.
Considering that carbon monoxide is one of the most commonly identified compounds in interstellar ices and ethanol is abundant in the ISM and has been tentatively detected in interstellar ices, lactaldehyde and its isomers could have formed upon exposure to GCRs in interstellar ices composed of carbon monoxide and ethanol within a cold molecular cloud. Once formed, these organics are promising candidates for future astronomical searches via radio telescopes. Notably, 3-hydroxypropanal has already been detected toward two star-forming regions.
Lactaldehyde is a key intermediate in the methylglyoxal pathway in biochemistry, where glucose is converted into pyruvate. Under prebiotic conditions, lactaldehyde acts as a fundamental precursor to essential biomolecules such as amino acids, sugars, and sugar acids. It can be oxidized to form methylglyoxal (CH3C(O)CHO), which further leads to the formation of pyruvic acid. Additionally, oxidation of lactaldehyde forms lactic acid (CH3CH(OH)COOH), eventually yielding sugar acids such as glyceric acid (HOCH2CH(OH)COOH). Through carbon-carbon or carbon-oxygen bond cleavage, lactaldehyde can be converted into glycolaldehyde (HOCH2CHO) or propionaldehyde (CH3CH2CHO). Once formed within cold molecular clouds, these organics could be incorporated into planetesimals and later delivered to planets, such as early Earth, providing an exogenous source for the synthesis of biorelevant molecules and offering valuable insights into the molecular origins of life4.
For further details, please refer to our article “Interstellar formation of lactaldehyde, a key intermediate in the methylglyoxal pathway,” published in Nature Communications.
Written by Dr. Jia Wang and Dr. Ralf I. Kaiser
References
1. Kleimeier, N. F., Eckhardt, A. K., Schreiner, P. R., Kaiser, R. I. Interstellar formation of biorelevant pyruvic acid (CH3COCOOH). Chem 6, 3385-3395 (2020).
2. McGuire, B. A. 2021 Census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules. Astrophys. J., Suppl. Ser. 259, 30 (2022).
3. Aponte, J. C., et al. Analyses of aliphatic aldehydes and ketones in carbonaceous chondrites. ACS Earth Space Chem. 3, 463-472 (2019).
4. Chyba, C., Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355, 125-132 (1992).
5. Turner, A. M., Kaiser, R. I. Exploiting photoionization reflectron time-of-flight mass spectrometry to explore molecular mass growth processes to complex organic molecules in interstellar and solar system ice analogs. Acc. Chem. Res. 53, 2791-2805 (2020).
6. Wang, J., et al. Interstellar formation of lactaldehyde, a key intermediate in the methylglyoxal pathway. Nat. Commun. 15, 10189 (2024).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in