From a new method to a new mechanism: the Long March to understand how islet insulin secretion is regulated by glucose stimulation
Published in Protocols & Methods, Cell & Molecular Biology, and General & Internal Medicine

Ever tried. Ever failed. No matter. Try again. Fail again. Fail better. ― Samuel Beckett
Part 1: The Journey
Act one: a promising start in imaging islets
During the first decade of the 21st century, insulin secretion research predominantly relied on three techniques, electrophysiology (patch clamp), molecular biology, and Total Internal Reflection Fluorescence microscopy (TIRFM). With the application of these techniques, many fundamental questions seem to be resolved. For example, the identification of readily releasable pool (RRP) and reserved pool (RP) of insulin granules have been linked to the glucose-stimulated biphasic insulin secretion, via a series of electrophysiology, electron microscopy, and genetical knockout experiments 1–6. Single-cell imaging using TIRFM has also provided complementary insights into the process of how individual granule fusions are regulated by glucose and other environmental factors 7–9. Despite these advancements and the seemingly well-correlated data, we still do not know how insulin secretions occur in islets and how they are coordinated.
About 13 years ago, I was a junior associated investigator in the Institute of Biophysics. Equipped with biophysics training and TIRFM imaging technique, I was trying to find a new way out to the old problem ― how glucose-regulated insulin secretions are regulated. It came to me that since insulin and zinc are secreted concomitantly, we may use an impermeable zinc dye to monitor insulin granule exocytosis from intact islets under the spinning disc confocal microscope. After the first round of trials by Lifeng Wang, a Ph. D student at that time, it seemed to work ― many fluorescence puncta emerged after the glucose stimulation. Most interestingly, it seemed that insulin secretions within the islet were not homogenous! Although we did not know how to analyze the data yet, an automated fusion detector for TIRFM developed by Yongdeng Zhang might resolve the problem10. Everything seemed rosy and promising, and I expected we might bolt down to the heart of the problem and finish it in three years. However, Dr. Lifen Wang graduated and moved to the United States to pursue postdoc training several months later. I moved to Peking University to start my own lab there. That changed everything. When Dr. Lu Yang joined my lab as a postdoc, and Xiaohong Peng came to the lab as a fresh graduate, the two key persons involved in the project knew little what a challenging journey lay ahead of this promising project.
Act two: a hard re-boot to obtain consistent results
Shortly after the leadership transition, we faced a significant setback when the original spinning disc microscope malfunctioned. This prompted Dr. Lu Yang and student Xiaohong Peng to switch to an alternative spinning disc microscope that had been configured for research on C. elegans. From that point forward, we were mired in constant challenges, struggling for several years to replicate the assay consistently. The results were perplexing: at times, we would observe hundreds of fusion events within a single islet; yet more often, there were none or only a few to record.
Initially, I thought this must be due to Xiaohong's inexperience in handling islet dissection, cultivation, and microscope operation. Therefore, she spent considerable effort refining the islet isolation and culture conditions, including optimizing various parameters, such as collagenase type, digestion time, purification protocols, hand-picked skills, and culture media composition. Despite these efforts to improve the islets' functional viability – evidenced by a robust Ca2+ signaling response to glucose – the secretion reproducibility remained unchanged.
Therefore, we surmised that additional factors might influence our results, including the adhesion of β cells and their proportion within the field of vision (VOF). After almost two years of diligent investigation, Xiaohong made a pivotal discovery well-known to other β cell researchers: temperature is a critical determinant in the success of our experiments. Consistent results were achieved only when assays were conducted at solution temperatures within the narrow range of 35-37°C. Our initial equipment had met this stringent temperature requirement thanks to a comprehensive heating system encompassing the entire microscope within a heated enclosure. However, after our original microscope became inoperative, the subsequent microscopes we used, which included those from other research groups and those we purchased, only featured a localized heating system with a smaller incubator. This limited temperature control led to solution temperatures that fluctuated too easily, yielding instability in our assays. However, when we tried to warm the entire room to maintain an appropriate temperature, the laser units within the microscopes began to shut off as a protective response against overheating frequently. To prevent operating continuously in the elevated ambient temperatures, we had no choice other than limiting the experimental hours in a day. The number of fusion events observed in a single islet proved susceptible to a confluence of variables: the solution temperature, islet integrity, the degree of cell attachment, and the proportion of β cells within the field of view, among others, contributed to a pronounced batch effect. To mitigate this variability, we aimed to collect data from ten pancreatic islets per day. However, the heat buildup within our working environment directly conflicted with this objective, as it perturbed the delicate conditions needed for our assays.
Ultimately, with the support of student Renjie Zhou last year, we finally engineered a final solution: a bespoke, real-time temperature-controlled enclosure for the microscope that concurrently allowed for continuous monitoring of the solution temperature. This customed device empowered us to achieve a consistent reproduction of the assay by different researchers, significantly decreasing the variability that had long challenged our efforts.
Act three: a twist-and-turn quest to analyze the data
Images are high-dimensional data. Therefore, sensitive and accurate computer-assisted programs to analyze large image data sets are necessary. For Xiaohong and Lu Yang, who were primarily trained as biological researchers, a significant challenge was the processing and analysis of image data, particularly upon identifying fusion events. The impermeable nature of the zinc probe meant that fusion events could only be observed once the insulin vesicles merged with the plasma membrane, a process accompanied by a marked increase in the fluorescence of punctate structures.
Given that these characteristics bear resemblance to VAMP2-pHluorin-associated fusion events imaged by TIRFM, Lifen Wang leveraged a customed program originally crafted by Dr. Yongdeng Zhang to detect VAMP2-pHluorin related fusion events in INS-1 cells as captured via TIRF microscopy10. In the beginning, Lifen relied exclusively on this program to identify fusion events without manual intervention. Unfortunately, Xiaohong, a novice in the field, could not replicate previously achieved results and naïvely noticed that the program faltered, particularly with tracking moving fusion events. During the troubleshooting phase, we began to uncover more significant issues with the program. For example, many fusion events within the islet under the spinning disc confocal came along with no spreading and the movement of fluorescence puncta, which differed significantly from VAMP2-pHluorin labeled fusion under the TIRFM. Additionally, the algorithm frequently misclassified noise that fulfilled the criteria set for actual fusion events. This led to a decline in accuracy, dipping below 70% in certain experiments, such as during GLP-1 stimulations. After quite a few trials to further process the images with little success in improving the accuracy, we manually checked all possible fusion events in the end, which constituted a huge requirement on human labor and a test of their perseverance.
In 2016, I learned that deep-learning algorithms may address the aforementioned issues. We were fortunate to enlist a master's student, Yi Wu, who undertook the development of a deep-learning-based algorithm. He leveraged our meticulously curated dataset comprising 100,000 hand-picked fusion event examples. After about a year, Yi Wu successfully created a long short-term memory (LSTM) model that pinpointed all possible fusion events (but never published), elevating the accuracy rate to an impressive 95%. At that juncture, the challenge of accurately identifying fusion events appeared to have been effectively resolved. However, in 2019, when we replaced the EMCCD with a CMOS camera for higher resolution confocal imaging, the LSTM algorithm failed again due to the smaller pixel size (160 nm to 65 nm), the application of a new Zn2+ probe with higher signal-to-noise ratio, and reduction in sampling rates (4Hz to 1Hz).
This was when Huixia Ren, a PhD student from an applied mathematics background, joined the team and started to address the question again by combining the principles of the first two algorithms. This new algorithm operated using a sliding window (3x3 pixels) to detect rapid shifts in fluorescence intensity. Yet, while it was adept at recognizing full fusion events, it fell short by redundantly marking the same events in cases of ongoing fusion activities. Although sensitive to fluctuations in fluorescence, the algorithm was markedly inaccurate when confronting moving exocytotic events. We still had to check possible fusion events one by one with only an average 50% accuracy ratio. This was the condition even when we submitted the paper to Nature Metabolism in May 2022.
In 2022, the revision process necessitated multiple new experiments that required dual-color imaging, and a more sensitive Zn2+ probe was used to detect secretions from islets isolated from disease mice. In addition, we needed to re-analyze some of the old images. This was the point that neither existing algorithm was adequate in processing this varied set of image data. At this crucial juncture, we benefited from the expertise of Haocheng Long, another graduate student, who was devoted to developing deep learning (DL) algorithms for image denoising. He skillfully adapted an existing DeepCAD-RT algorithm 11 to our needs and used the Laplacian of Gaussian (LoG) operator to detect the fluorescence puncta. The new algorithm allowed us to identify fusion events across various image datasets with high accuracy and minimal variations, signifying that denoising is pivotal.
Part 2: The Insights
Concept 1: the RRβ cells and the need for a holistic view of biphasic GSIS
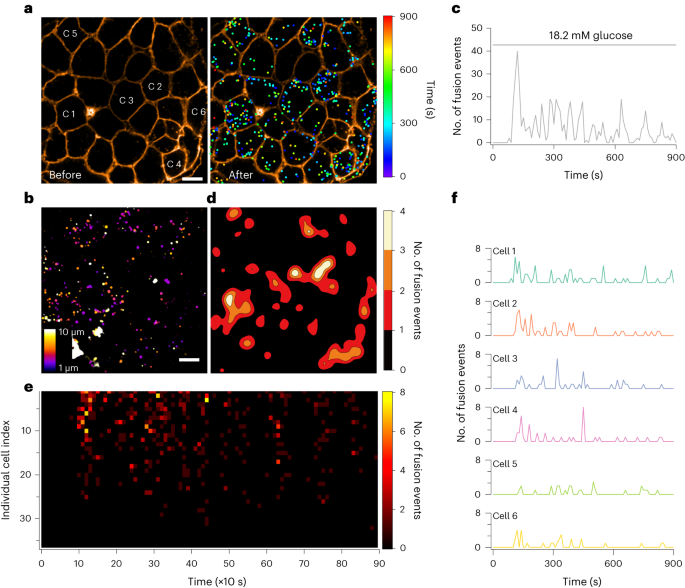
The project was initially kick-started with an imaging method to detect insulin secretions within the intact islet. Once the data were visible, we soon realized substantial heterogeneity in the glucose-stimulate insulin secretion (GSIS) from individual β cells within the islet. However, it was not proved until Huixia made quite some effort to devise different mathematical tests. For example, she found that the secretory capacity of individual β cells within the islet always displayed exponential function. Moreover, she extracted the Gini Coefficient from the relationship between the cumulative cell number and their fusion events. Most of the time, Gini Coefficients of islet secretions stimulated by glucose were often around 0.5, approximating an ideal exponential distribution. Finally, she also designed Voronoi and sub-Voronoi diagrams to prove the spatial heterogeneity of islet exocytotic events. These data suggest that only a small proportion of β-cells release insulin within the islet upon glucose stimulation. Thus, we proposed the concept of readily-releasable β-cells (RRβ), which comprised often 40% of all islet β cells but contributed to 80% of all secretion. Our islet Zinc imaging method enables us to measure the total islet secretion dynamics and distinguish the contributions from individual b cells simultaneously. Astonishingly, our analysis revealed that the initial and subsequent phases of GSIS rely exclusively on the same pool of RRbs. Meanwhile, the remaining β cells appear to remain inactive, not taking part in insulin release during glucose stimulation.
It is long believed that the exocytosis of RRP and RP insulin granules correspond to the first and the second phases of GSIS. In particular, the knockout of CaV1.2 or Cav2.3 inhibited the exocytosis of RRP or sustained exocytosis in single β-cells stimulated by a train of depolarization. Simultaneously, either the first or the second phase of GSIS from the islet was compromised. The supplementary of either molecule readily rescued the specific exocytosis defective phenotype in single cells and islets, closing the loop of the conventional definition of molecular mechanism. Manipulation of other molecules, such as Munc18-1 and Munc13-1, also reinforced this line of interpretation12,13. However, it was only through the simultaneous examination of insulin secretion at both the single-cell and the collective islet levels that we realized the central regulatory mechanism of GSIS operates primarily at the islet level, rather than at the level of granules within individual cells. The coordinated exocytosis from RRβs creates the fast initial phase of GSIS, while their later, staggered secretion defines the slower second phase.
Thus, the intricate mechanism underpinning the biphasic nature of GSIS cannot be solely attributed to specific molecules, a notion often favored by molecular biologists who advocate for reductionism. Instead, the unique biphasic characteristic of GSIS emerges as a collective property of individual α, β, and δ cells functioning together within the islet complex.
Concept 2: Surprisingly decoupled Ca2+ and insulin secretion from islet β-cells
The discovery of RRβs has prompted a closer investigation into the nature of Ca2+ signals within these cells. Understanding this is crucial since distinct Ca2+ channels have been linked to the various phases of GSIS5,6. Furthermore, researchers have characterized islet b cells based on Ca2+ dynamics into various categories such as “first responders,” “second followers,” “leaders,” and “hub cells”14–16. However, imaging Ca2+ alongside secretion simultaneously presents a significant challenge due to the absence of a sensitive, non-toxic Zinc dye compatible with green Ca2+ indicators like Ins-Gcamp6f. This situation persisted until Dr. Zhixing Chen, an expert chemist, joined our institute in 2018. Dr. Chen sought to create improved chemical dyes for bioimaging purposes. During an informal lunchtime discussion, we explored various potential collaborative projects. Among these, the idea of a high-performance red Zinc dye that boasted excellent water solubility and a strong signal-to-noise ratio stood out. Yunxiang Wu, a skilled technician in Dr. Zhixing Chen's lab, invested ten months and underwent two iterative development cycles to create various prototypes of red and far-red Zinc dyes. Our perseverance was rewarded when fortune smiled upon us — one variant of the red dye proved to be a perfect match for our system, yielding vivid signals that beautifully captured insulin secretion in tandem with the Ca2+ signaling monitored by Gcamp6f.
Thereafter, Xiaohong worked hard to collect data from many islets with insulin secretion and Ca2+ simultaneously recorded. Then Huixia analyzed the data. Working collaboratively, they endeavored to decipher the correlation between Ca2+ dynamics and secretion activity and also investigated the existence of potential hub or leader cells as earlier hypothesized — however, they found no evidence of such cells. Additionally, it appeared that there was no evident correlation between various parameters of the Ca2+ signals, such as their amplitude or initiation speed, and the insulin secretion rates of individual islet β cells. Specifically, RRβ and RIβ cells in the islet exhibited nearly identical Ca2+ signals when stimulated by glucose, yet their secretion rates varied by several magnitudes. This is in sharp contrast to the isolated β cell studies conducted previously.
Accordingly, we drew inspiration from the concept of coupling used in neuroscience and cardiovascular biology. We observed that the close coupling between Ca2+ signaling and insulin secretion, seen in RRβ cells, was absent in RIβ cells. Therefore, even though Ca2+ signals act as a uniform trigger across all β cells, the actual secretion of insulin from these cells hinges on the presence and efficacy of the coupling, especially in islets and pancreas.
Lessons learned: Biology is always more complex than we thought
The apparent inconsistencies in coupling efficiency may be attributed to a range of paracrine regulatory factors, as extensively reported in preceding literature within this field17–21. In particular, we believe that RIβ cells may be clamped by the d cells in the neighborhood. However, the process of confirming these reports was fraught with complications. In particular, introducing the same disturbance often led to unpredictable experimental outcomes. For instance, the application of glucagon antagonists yielded inconsistent effects on the exocytosis of insulin granules, alternately inhibiting or having no discernible impact. Similarly, manipulation of SSTR2 function using CYN also resulted in various divergent responses.
One evening, through serendipity, while analyzing data from Stdt mice wherein islet δ-cells were marked with tdTomato, Xiaohong experienced an epiphany. She postulated that islets could harbor varying amounts of δ cells. With focus attention, she enumerated the δ cells across the focal plane and the entire cellular volume and scrutinized their degree of clustering. By incorporating these data, Xiaohong speculated that the varied reactions to antagonists might be related to the quantities of α and δ cells in the islet and their spatial interplay. Along with this line of thought, our research utilizing the ob/ob mouse model has revealed numerous alterations in islet β-cell Ca2+ signaling and secretion correlated with the progression of the disease. Overall, considering the islet as a complex microcosm, teeming with diverse cell types, intricate interactions, and dynamic activities, it may not be possible to isolate a singular definitive piece of evidence — the 'smoking gun' — that explains the full spectrum of processes occurring within the islet.
People involved in the project:
|
Name |
At the time joined the project |
Position then |
Current position |
|
Xiaohong Peng |
2011 |
PhD student |
Technician |
|
Huixia Ren |
2016 |
PhD student |
Postdoc |
|
Lu Yang |
2011 |
Postdoc |
Assistant investigator |
|
Shiyan Tong |
2019 |
PhD student |
High school teacher |
|
Renjie Zhou |
2022 |
PhD student |
PhD student |
|
Haochen Long |
2022 |
Master student |
Master student |
|
Yunxiang Wu |
2018 |
Technician |
PhD student at UW Madison |
|
Lifen Wang |
2010 |
PhD student |
Researcher in a US pharmaceutical company |
|
Yi Wu |
2016 |
Master student |
Senior engineer at Huawei |
|
Yongdeng Zhang |
2011 |
PhD student |
Tenure-track Assistant Professor at the University of Westlake |
|
Jiayu Shen |
2018 |
Master student |
Senior engineer at Huawei |
|
Junwei Zhang |
2020 |
PhD student |
PhD student |
|
Guohua Qiu |
2022 |
PhD student |
PhD student |
|
Jianyong Wang |
2021 |
Master student |
Engineer in Momenta |
|
Chengsheng Han |
2014 |
PhD student |
Senior industry analyst |
|
Yulin Zhang |
2015 |
PhD student |
Researcher in a Pharmaceutical company |
|
Mengxuan Zhou |
2017 |
Master student |
Venture Capital Investment fund senior associate |
|
Yiwen Zhao |
2021 |
PhD student |
Postdoc |
|
Tao Xu |
2010 |
Professor and Director of the Institute of Biophysics, CAS |
Academician of CAS, Deputy Director of Guangzhou Laboratory |
|
Chao Tang |
2016 |
Professor |
Academician of CAS, Director of CLS Center in Peking University |
|
Zhixing Chen |
2018 |
Tenure-track Assistant Professor |
Tenure-track Assistant Professor in Peking University |
|
Huisheng Liu |
2018 |
Professor at Beihang University |
Professor in Guangzhou Laboratory |
|
Liangyi Chen |
2010 |
Associated Investigator in Institute of Biophysics, CAS |
Tenured Professor in Peking University |
Where I am, I don’t know, I’ll never know, in the silence you don’t know, you must go on, I can’t go on, I’ll go on. ― Samuel Beckett
Reference:
- Barg, S. & Eliasson, L. A Subset of 50 Secretory Granules in Close Contact With L-Type Ca2+ Channels Accounts for First-Phase Insulin Secretion in Mouse b-Cells. 51, (2002).
- Rorsman, P. et al. The Cell Physiology of Biphasic Insulin Secretion. Physiology 15, 72–77 (2000).
- Rorsman, P. & Renstrom, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 46, 1029–1045 (2003).
- Olofsson, C. S. et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflüg. Arch. 444, 43–51 (2002).
- Schulla, V. et al. Impaired insulin secretion and glucose tolerance in β cell-selective CaV1.2 Ca2+ channel null mice. EMBO J. 22, 3844–3854 (2003).
- Jing, X. et al. CaV2.3 calcium channels control second-phase insulin release. J. Clin. Invest. 115, 146–154 (2005).
- Seino, S., Shibasaki, T. & Minami, K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. https://www.jci.org/articles/view/45680/pdf (2011) doi:10.1172/JCI45680.
- Ohara-Imaizumi, M. et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J. Cell Biol. 177, 695–705 (2007).
- Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. https://www.pnas.org/doi/10.1073/pnas.0707054104 doi:10.1073/pnas.0707054104.
- Yuan, T., Lu, J., Zhang, J., Zhang, Y. & Chen, L. Spatiotemporal Detection and Analysis of Exocytosis Reveal Fusion “Hotspots” Organized by the Cytoskeleton in Endocrine Cells. Biophys. J. 108, 251–260 (2015).
- Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit | Nature Biotechnology. https://www.nature.com/articles/s41587-022-01450-8.
- Kang, L. et al. Munc13-1 is required for the sustained release of insulin from pancreatic β cells. Cell Metab. 3, 463–468 (2006).
- Oh, E., Kalwat, M. A., Kim, M.-J., Verhage, M. & Thurmond, D. C. Munc18-1 Regulates First-phase Insulin Release by Promoting Granule Docking to Multiple Syntaxin Isoforms. J. Biol. Chem. 287, 25821–25833 (2012).
- Salem, V. et al. Leader β-cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nat. Metab. 1, 615–629 (2019).
- Johnston, N. R. et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 24, 389–401 (2016).
- Kravets, V. et al. Functional architecture of pancreatic islets identifies a population of first responder cells that drive the first-phase calcium response. PLoS Biol. 20, e3001761 (2022).
- Noguchi, G. M. & Huising, M. O. Integrating the inputs that shape pancreatic islet hormone release. Nat. Metab. 1, 1189–1201 (2019).
- Rorsman, P. & Huising, M. O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat. Rev. Endocrinol. 14, 404–414 (2018).
- Capozzi, M. E. et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4, e126742.
- Koh, D.-S., Cho, J.-H. & Chen, L. Paracrine Interactions Within Islets of Langerhans. J. Mol. Neurosci. 48, 429–440 (2012).
- Hauge-Evans, A. C. et al. Somatostatin Secreted by Islet δ-Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Diabetes 58, 403–411 (2009).
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in