From Orphan Receptor to Metastatic Catalyst: Unveiling the Role of GPR107 in Breast Cancer Progression
Published in Cancer
Breast cancer metastasis continues to represent the primary challenge in reducing cancer-related mortality among women worldwide. While diagnostic and therapeutic advances have improved outcomes for localized disease, the prevention and treatment of disseminated disease remain a formidable clinical obstacle. The metastatic cascade is fundamentally dependent on the dynamic interplay between tumor cells and their microenvironment, with the extracellular matrix (ECM) serving as a critical barrier that must be breached for successful dissemination. Here, we identify G protein-coupled receptor 107 (GPR107), a previously uncharacterized orphan receptor, as a master regulator of this invasive process. We delineate a complete molecular pathway through which GPR107 coordinates the dismantling of collagen IV (COL4), a foundational component of the basement membrane, thereby enabling tumor cell escape and metastatic progression. Our findings not only reveal GPR107 as a potent promoter of breast cancer metastasis but also uncover novel therapeutic opportunities for targeting the metastatic cascade.
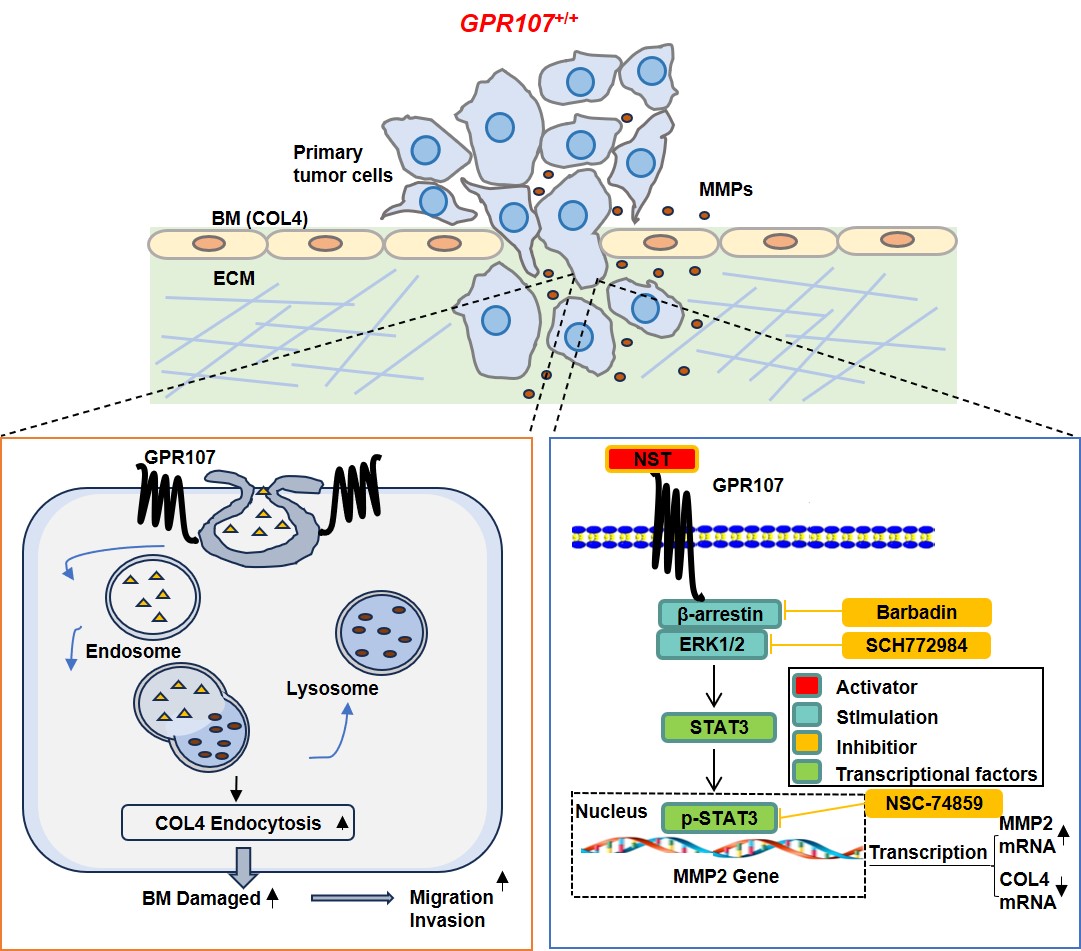
A Multi-Pronged Attack on Collagen IV
GPR107 orchestrates the removal of COL4 from the ECM through three distinct, synergistic mechanisms:
1. Enhanced Endocytosis: GPR107 facilitates the internalization of COL4 from the ECM via Clathrin-mediated endocytosis. This process actively scavenges and removes existing COL4 protein from the cellular surroundings.
2. Increased ECM Degradation: GPR107 signaling leads to a significant upregulation in the production of matrix metalloproteinase-2 (MMP2), a potent enzyme whose primary function is to degrade ECM components, including COL4. This represents an extracellular "scissors" mechanism, chewing up the collagen scaffold.
3. Suppressed Biosynthesis: GPR107 activity also suppresses the very production of new COL4. This indicates that it not only clears existing matrix but also halts its replenishment, ensuring the barrier remains compromised.
GPR107 operates through a β-arrestin-dependent activation of the ERK/STAT3 signaling cascade. This pathway activation drives MMP2 expression while simultaneously suppressing COL4 gene transcription, creating a unified molecular circuit for ECM destruction.
Translational Significance
Clinical analyses reveal GPR107 is overexpressed in breast carcinomas, with highest levels in metastatic lesions correlating with poor patient survival. Functional experiments demonstrated that manipulating GPR107 levels directly impacted the aggressive behavior of cancer cells; its expression was a key enabling factor for their ability to invade and spread. Functional validation demonstrates that GPR107 knockout suppresses liver metastasis in vivo, while its overexpression enhances metastatic burden. These findings position GPR107 as a prognostic biomarker for metastatic risk stratification, and a promising therapeutic target, with inhibition potentially restoring ECM integrity to prevent metastatic dissemination.
In conclusion, our work elevates GPR107 from an orphan receptor to a central regulator of breast cancer metastasis. By unveiling its multi-faceted attack on collagen IV and delineating the underlying β-arrestin/ERK/STAT3 mechanism, we provide a new framework for understanding tumour cell invasion. We propose that targeting GPR107 represents a fertile and previously unexplored frontier for developing innovative strategies to predict, prevent, and treat metastatic breast cancer.
Follow the Topic
-
Cancer Gene Therapy

The essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in