From PEG to ion-pair network: the “magic crosslinking” behind a steric stabilization-independent stealth cloak
Published in Bioengineering & Biotechnology, Cancer, and Materials
The “how” behind a stealth cloak
For several decades, the dominant recipe for “stealth” nanoparticles has sounded deceptively simple: add a soft, hydrated polymer brush (like PEG) to keep proteins and immune cells at bay. But as anyone who has tried to precisely tune PEG density, chain length, and conformation learns quickly, the real world is messier. Many “stealth” designs still show rapid first-phase (α-phase) blood clearance, a sign that nano-bio interactions remain stubbornly active.
Our team took a different approach. Rather than maximizing steric repulsion, we asked: what if we minimize the driving force for nano-bio binding altogether? That question led us to what we call “magic crosslinking” — not magic in a mystical sense, but because a small structural shift suddenly flipped the entire system’s behavior.
The moment the curve stopped dropping
Working with polyion complex (PIC) micelles (~30 nm) and vesicles (~100 nm), we gradually crosslinked the oppositely charged polymer chains forming their cores. On paper, crosslinking simply ties neighboring chains together. In blood, something extraordinary happened: once crosslinking passed a critical threshold (~40 % for micelles and ~30 % for vesicles), the nanoparticles stopped doing what every nanoparticle normally does at early time points — undergo rapid immune clearance and bleed out of circulation.
Above that threshold, intravital microscopy showed a stable blood signal for hours, and pharmacokinetics revealed monophasic half-lives exceeding 100 hours. Below it, the same formulations vanished within minutes. The switch was abrupt — a hallmark of a percolation-type transition rather than a gradual effect. To us, it felt like flipping on a hidden stealth switch.
Why “magic” crosslinking works
At nano-bio interfaces, two entropic payoffs drive unwanted binding: the release of bound water and of counterions. Charged surfaces attract clouds of small ions, and when proteins approach, those ions are released — a process that rewards binding. But if opposite charges inside the nanoparticle pair up cooperatively and remain fixed, there are fewer ions and less water to release. Binding becomes thermodynamically unrewarding. Crosslinking produced three measurable changes:
- Network locking: NMR line broadening and T₂ shortening revealed reduced polymer mobility, forming a rigid, system-spanning ion-pair network.
- Reduced counterion occupancy: associated ions dropped precisely at the stealth threshold.
- Resistance to protein adsorption: fluorescence correlation spectroscopy and calorimetry showed nearly no protein binding, consistent with dramatically reduced macrophage uptake and long circulation.
The system transitioned from a dynamic cluster soup to a cooperative, percolated ion-pair sheath — a stealth born of internal cohesion, not fluffy polymer brushes.
This discovery also made us revisit a classic: PEGylated liposomes. Their zwitterionic phospholipid bilayer naturally forms headgroup ion pairs. At the low PEG densities used in many successful formulations, the brush is too sparse for strong steric repulsion. Our data suggest PEG may instead stabilize intermolecular pairing among phospholipid headgroups — tightening a surface inter-zwitterion network. Different materials, same principle: cooperative pairing at the interface.
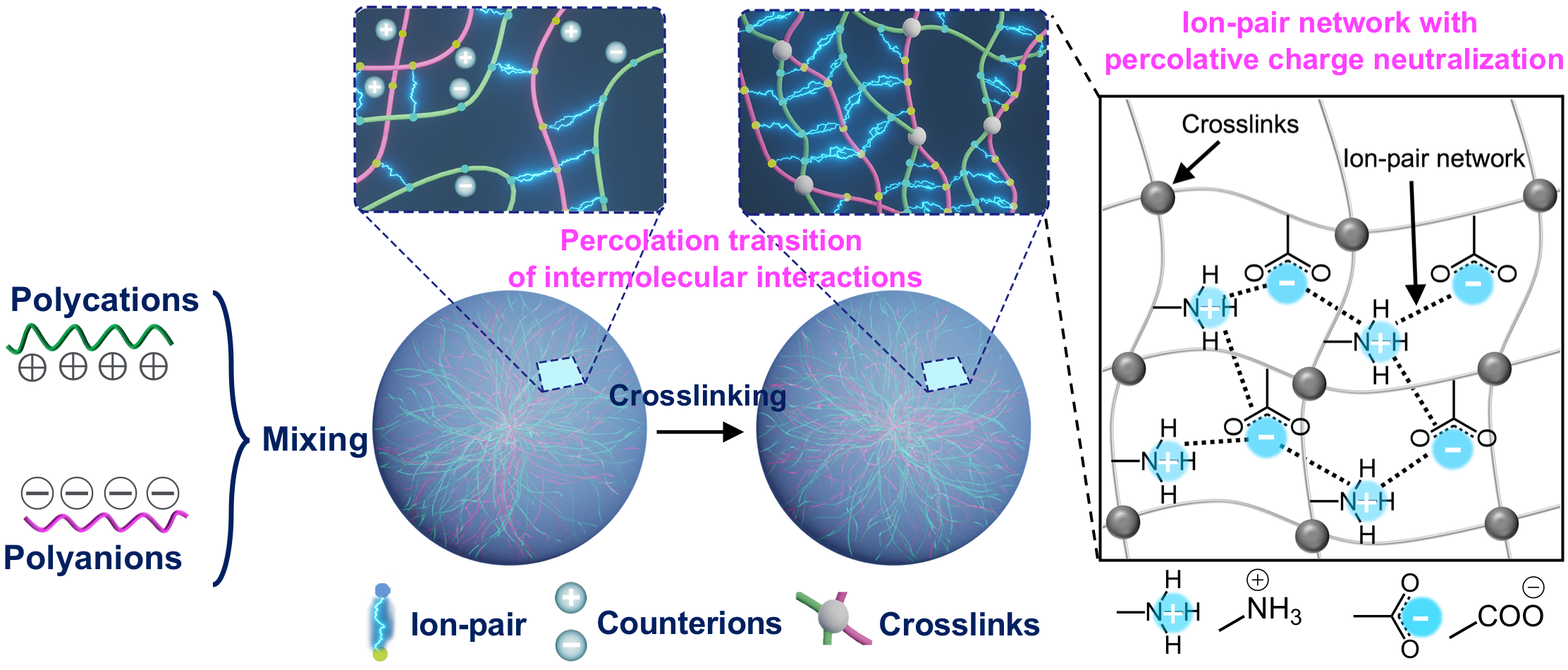
Schematic illustration of fabricating a stable ion-pair network with percolative charge neutralization by crosslinking
Turning a physics idea into a therapy
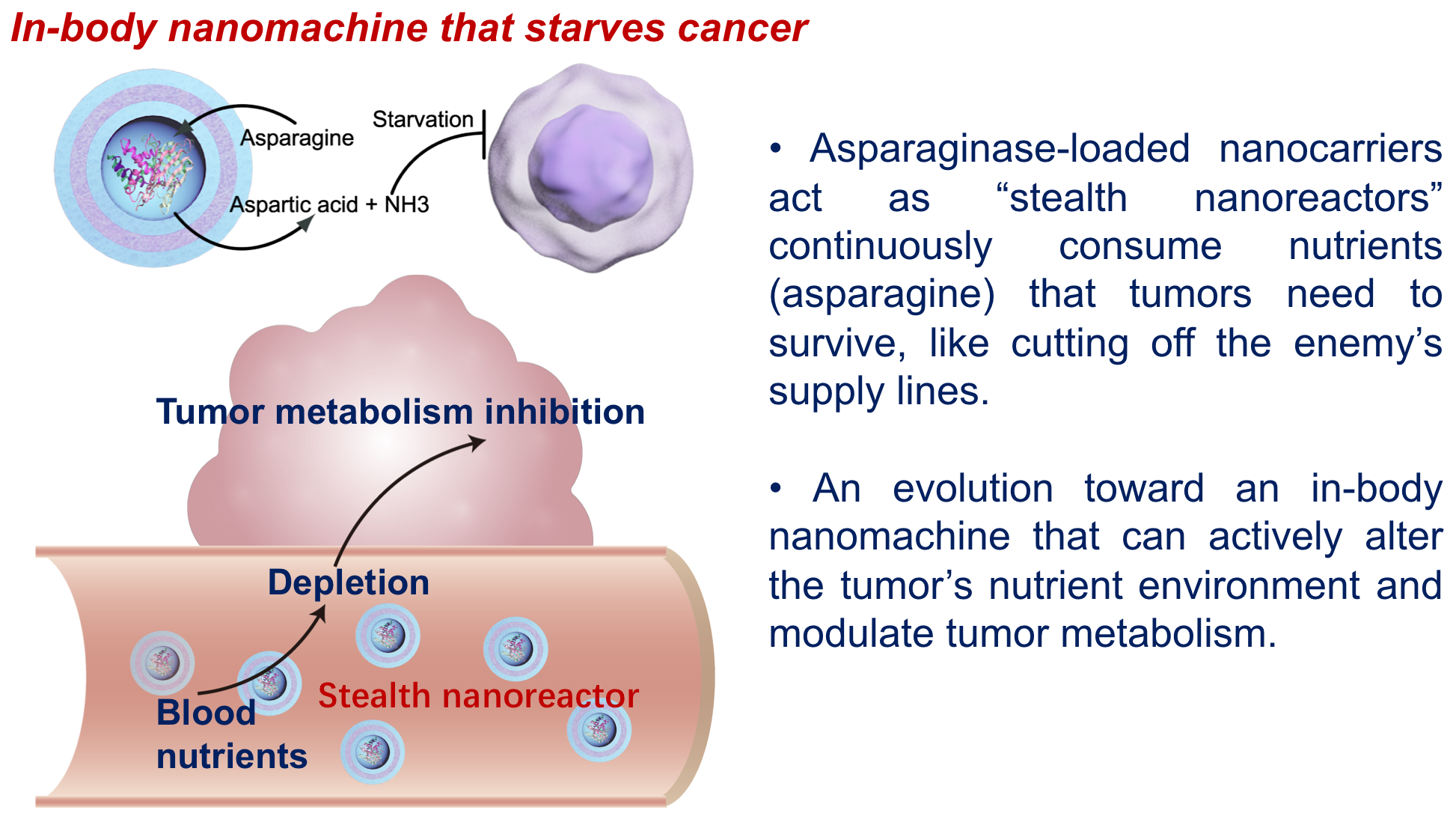
Long circulation is not the goal itself — it’s a means to make therapy last. We encapsulated the enzyme asparaginase inside the long-circulating PIC vesicles, creating a stealth nanoreactor with a semi-permeable sheath. Small nutrients such as asparagine can diffuse in and be consumed, while the enzyme stays protected. The goal was systemic asparagine depletion to starve tumors — a metabolism-first strategy rather than traditional “EPR-targeting”.
In metastatic breast cancer models (4T1 and 231/LM2), these nanoreactors achieved what free enzyme could not: sustained asparagine depletion with infrequent dosing and strong suppression of tumor growth and metastasis. We then moved to pancreatic ductal adenocarcinoma (PDAC) using the stringent KPC genetic model, known for its dense, fibrotic stroma and immune exclusion — conditions that make most drugs, even immunotherapies like anti-PD-1, ineffective. Despite their long circulation, ~100 nm vesicles could not easily penetrate PDAC tissue. Yet, prolonged asparagine depletion dramatically reduced collagen and cancer-associated fibroblast markers, alleviating desmoplasia. This microenvironmental softening immediately suggested a combination strategy: pair the nanoreactor with small (~15 nm) anti-PD-1 antibodies. After priming with the nanoreactor, anti-PD-1 penetrated deeper and accumulated more uniformly, producing strong tumor regression and extended survival. Metabolism reshaped the physical barrier — and immunity slipped through.
Summary and implications
Our journey began with a simple question—could we make nanomaterials invisible to biology without relying on polymer brushes? The answer emerged through what we called magic crosslinking, where a percolated ion-pair network sheath spontaneously conferred stealth behavior. This finding reshapes how we think about “non-fouling” surfaces. Instead of building thicker or denser polymer layers to push proteins away, we can now stabilize the interface itself, minimizing the entropic reward for binding.
Beyond materials design, the implications reach into therapy. The asparaginase-loaded stealth nanoreactor transformed long circulation into an active treatment, continuously depleting nutrients and starving tumors systemically. The idea is not “more drug in”, but “reshape the environment”.
In pancreatic cancer — among the most treatment-resistant malignancies — sustained asparagine starvation softened fibrotic stroma and opened a pathway for immune infiltration. Combined with anti-PD-1 immunotherapy, the effects were synergistic, revealing that metabolic modulation can re-engineer both physical and immune barriers.
Looking forward, this approach points to several directions:
- A new stealth design rule: cooperative ion-pair or inter-zwitterion networks can replace PEG in creating long-circulating, non-fouling nanoparticles.
- In-body nanomachines: enzyme-loaded vesicles function continuously, reshaping the tumor ecosystem rather than merely delivering drugs.
- Simpler translation: because it modulates systemic metabolism instead of relying on tumor targeting, the strategy may generalize across cancer types.
- Societal reach: long-acting enzyme carriers could bring “starvation therapies”, once limited to blood cancers, into solid tumors that have long resisted treatment.
In the end, what began as a question of physics has evolved into a bridge between materials science and medicine — showing how a subtle change in molecular order can redefine both stealth and therapy.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcement
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in