From Red Algae to Better Batteries: The Journey Behind Our Research
Motivation to study Li-S battery and binder materials
Nowadays, the vast majority of us use a wide range of portable electronic devices, perhaps with the thought that the manufacture of these devices today is associated with a large ecological footprint, for example because of the Li-ion batteries they contain. The production of Li-ion batteries requires conflict minerals and toxic solvents.
Lithium-sulfur (Li-S) batteries hold great promise for the future of energy storage, but their stability issues remain a challenge. In our latest study, we explored how a polysaccharide binder derived from red algae can significantly improve battery performance, making Li-S technology more viable for real-world applications.
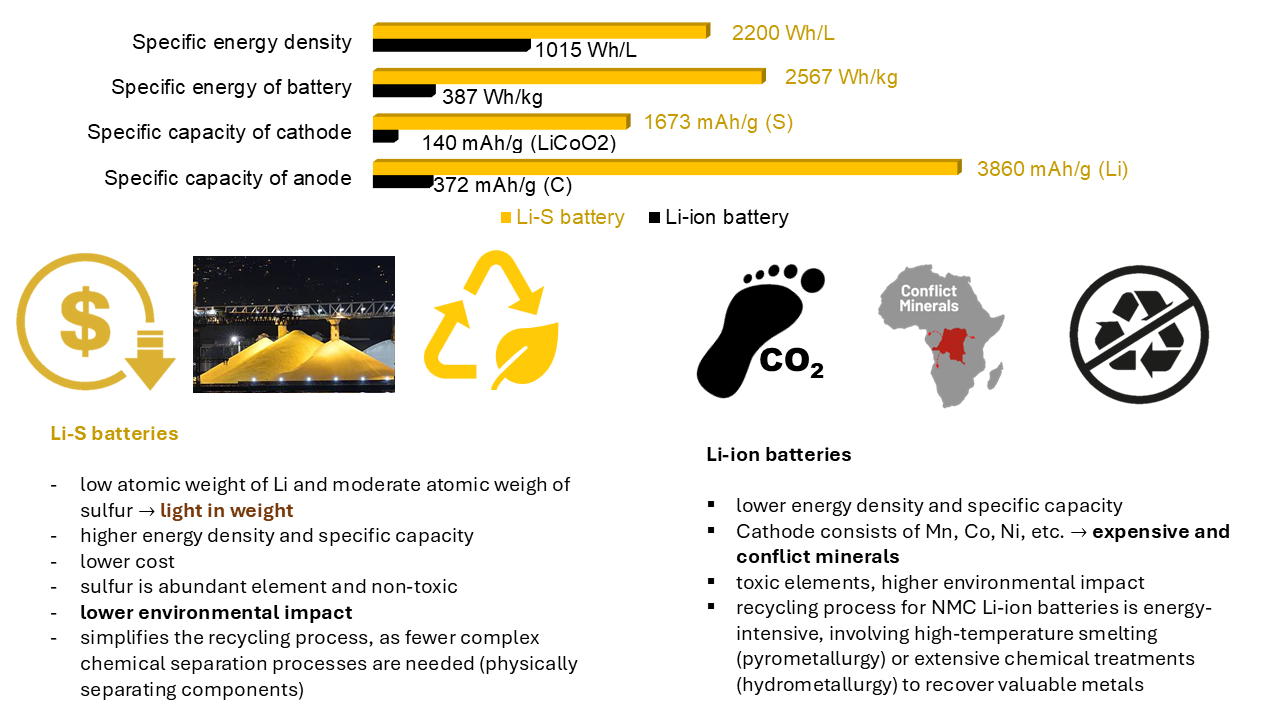
Figure 1 Comparison of some aspects of the Li-ion and Li-S technology
With the increasing demand for high-capacity, eco-friendly energy storage solutions, Li-S batteries have gained attention due to their high theoretical capacity and energy density. However, their commercial production has been hindered by major obstacles such as capacity loss over repeated cycles and structural instability caused by the expansion and contraction of sulfur electrodes during operation. Additionally, dissolved sulfur species can migrate within the battery, leading to further degradation over time. Addressing these challenges requires new approaches in electrode design.
One promising strategy is to overcome the volume change problems is modifying the binder material, which holds electrode components together. The most widely used binder, polyvinyl fluoride (PVDF), was originally adapted from lithium-ion battery technology. While PVDF provides strong physical adhesion, it lacks flexibility and does not help mitigate volume changes in Li-S batteries. Furthermore, it is hydrophobic material and requires the use of toxic organic solvents like N-methyl-2-pyrrolidone (NMP) during electrode preparation.
To overcome these limitations, we explored alternative polymeric binders that are both water-compatible and mechanically robust. We specifically sought materials that could not only provide strong adhesion but also contain chemical functional groups capable of interacting with sulfur species. Previous studies have examined for instance chitosan and gelatin, both of which show promise. However, we focused on carrageenan, a polysaccharide derived from red algae, because of its plant-based origin and the fact that it is often considered marine waste, making it an ecologically attractive option. Some literature source [1], [2] suggested that its sulfate groups might facilitate chemical interactions with lithium polysulfides, but others disputed this claim [3]. Our goal was not just to apply a more environmentally friendly material, but to deeply investigate its electrochemical behavior and determine whether its unique chemical properties contributed to performance improvements. At the same level, it is important how much of a given binder we should put into the cathode to make it work optimally. That's why we also looked at the optimal ratio of the binder material that gives the best results. In addition, we wanted to understand why and how carrageenan could improve battery performance.
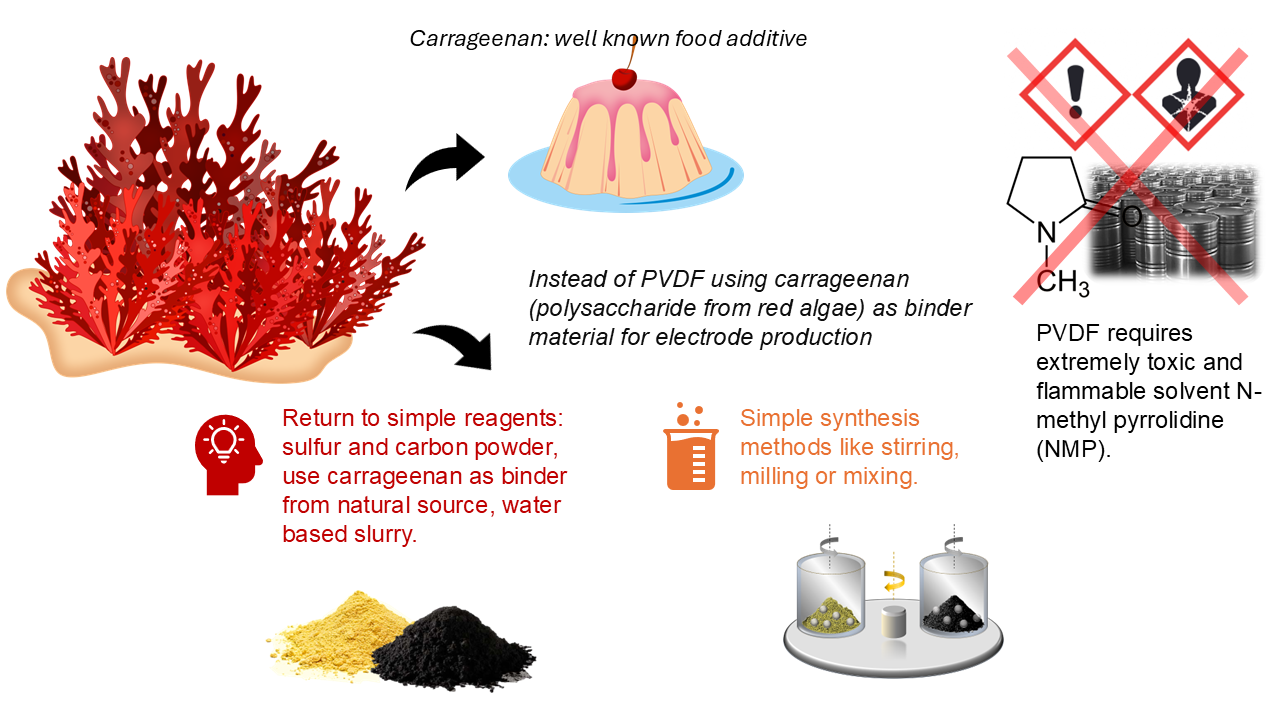
Figure 2 Illustration of the ecological advantages of using carrageenan binder material instead of the PVDF
It is just a cherry on the top of the cake that carrageenan is widely used as a food additive, often found in dairy products and baked goods as gelling component. This dual functionality made it even more fascinating to explore its role in battery chemistry.
What did the authors do? – A cooperation between Budapest (Hungary), Kosice (Slovakia), Ljubljana (Slovenia) and Krakow (Poland).
We set out to understand whether carrageenan could enhance battery performance by improving sulfur retention and stabilizing electrode structure. The first step was designing electrodes using a water-based method, eliminating the need for toxic solvents like N-methyl-2-pyrrolidone (NMP). This work was started at Kosice and Budapest (by Dóra Zalka, Andrea Strakova Fedorkova, Lakshmi Shiva Shankar, Róbert Kun and Karel Saksl).
The electrochemical tests were promising: electrodes with carrageenan achieved 30% higher capacity than those with PVDF and maintained over 69% of their initial capacity after 1000 charge-discharge cycles at 4C. This was a significant improvement in cycle stability, suggesting that carrageenan binds sulfur effectively and mitigates polysulfide loss.
To further investigate the mechanism, we employed scanning electron microscopy (SEM, Zoltán Dankházi, Budapest) X-ray photoelectron spectroscopy (Zoltán Pászti, Budapest) and operando X-ray absorption spectroscopy (XAS, SOLARIS, Kraków, Dóra Zalka, Alexey Maximenko and Pál Jóvári) involving researchers from Ljubljana, National Institute of Chemistry (Alen Vižintin). This international cooperation and these techniques allowed us to track sulfur species during battery operation and confirmed that carrageenan chemically interacts with polysulfides, preventing their dissolution into the electrolyte. Unlike PVDF, which merely acts as a passive component and a physical binder, carrageenan plays an active role in stabilizing the battery chemistry.
Challenges and surprises along the way
Research often comes with unexpected challenges, and our journey was no exception. One of the most difficult aspects was designing X-ray absorption measurements at the sulfur K-edge. At such low energies, where sulfur can be measured, even a few centimeters of air could block the X-rays completely. We had to develop specialized in-operando cells and experiment with different setups to ensure accurate transmission measurements.
There were also moments of trial and error in handling delicate samples. First, we wanted to investigate Li-polysulfides with XAS technique as liquids but UV-VIS spectroscopy revealed that these samples are easily decompose and oxidized even after 24 or 48 hours (Kristóf Hegedüs, Budapest). In one instance, we placed reference samples in a pouch cell with two Mylar films. However, as soon as we evacuated the chamber to 24 torr which is necessary to obtain an appropriate absorption signal, the pellets broke between the films, rendering the experiment unusable. After multiple attempts, with my colleagues finally we found a viable solution that allowed us to collect meaningful data, we closed them between two Kapton tapes. But it was also important to use such kind of Kapton tape which does not contain any sulfur and silicon. So one of my first task at synchrotron was to measure the XRF spectra of multiple Kapton tapes to select the proper one.
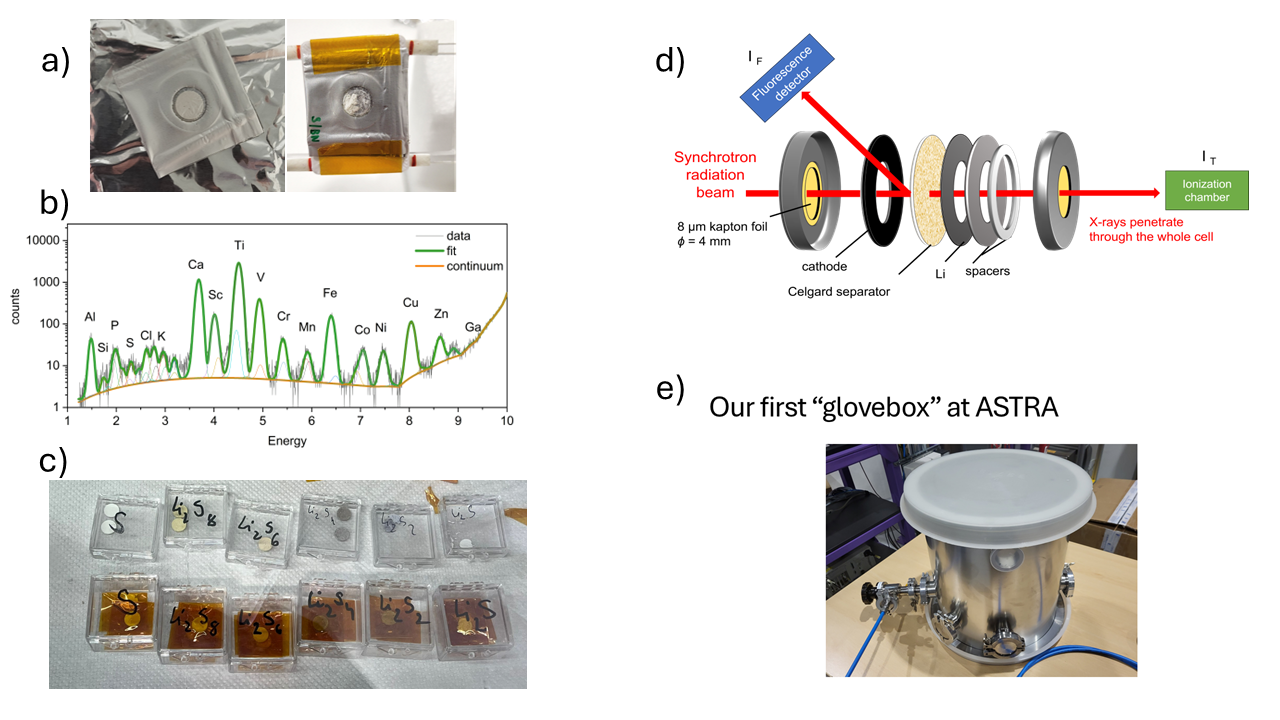
Figure 3 a) pellets sealed between two mylar foils and the broken pellet after evacuation of the sample chamber, b) XRF spectrum of the sulfur-free Kapton tape, c) Li-polysulfide standards closed between the sulfur-free Kapton tape, d) CR2032 cell modified for transmission measurements, e) our first “glovebox” at ASTRA beamline where we used the basic principle of physics that argon is heavier than air.
Beyond technical difficulties, there was the challenge of balancing the electrochemical and XAS measurements. What worked well in terms of collecting X-ray signal often had drawbacks in electrochemical performance. It took several iterations before we optimized the system both stability and preserve the electrochemical integrity of the battery.
Another aspect of this research was that we were the first people in the SOLARIS synchrotron to measure Li-S batteries. This made the process even more exciting, as with Alexey Maximenko (Beamline responsible at ASTRA beamline of SOLARIS) we were pioneering new techniques and pushing the boundaries of what was previously possible, especially in convincing Safety Group that we will not blow up the synchrotron and there will be still measurements possible once the experiment will be done. In addition, initially, we didn’t have too much tools for the measurements, so for instance we needed to figure out how to work at the synchrotron environment with air-sensitive samples without using glove box. Knowing basics of physics helped us a lot, where the samples were handled using the principle that more heavy gas (argon) is concentrated on the bottom of the chamber. It took lot of planning and work from our side and having the moto “No pain – no gain” we often worked 24 hours with no rest.
.jpg)
What are the implications of this study?
Our study highlights the often-overlooked role of binders in battery performance. Traditionally, binders have been seen as passive components, but our findings highlight that with the right material, in the proper ratio, they can actively contribute to improving the electrochemical stability of Li-S batteries.
The use of biomass-derived materials like carrageenan opens up possibilities for more sustainable and environmentally friendly battery manufacturing. Moving forward, we hope that our research inspires further exploration of alternative binders and their interactions with battery chemistries.
While our work demonstrates significant improvements, challenges remain before Li-S batteries can be fully commercialized. Further studies are needed to optimize large-scale production and ensure compatibility with different battery architectures. However, our findings represent a crucial step toward making Li-S technology more feasible for real-world applications.
Conclusion: Scientific research is rarely a straight path—it's a journey filled with unexpected obstacles, late nights, and countless iterations. But every breakthrough, no matter how small, brings us closer to solving real-world problems. Our work with carrageenan-based binders is just one piece of the puzzle in advancing Li-S batteries, but we believe it has the potential to make a meaningful impact in the quest for better energy storage solutions.
Read our full study here: Improving lithium-sulfur battery performance using a polysaccharide binder derived from red algae | Communications Materials
References:
[1] Ling, M. et al. Nucleophilic substitution between polysulfides and binders unexpectedly stabilizing lithium sulfur battery. Nano Energy 38, 82–90 (2017).
[2] Kazda, T. et al. Carrageenan as an ecological alternative of polyvinylidene difluoride binder for li-s batteries. Materials 14, 5578 (2021).
[3] Blanchard, D. & Slagter, M. In operando Raman and optical study of lithium polysulfides dissolution in lithium–sulfur cells with carrageenan binder. J. Phys. Energy 3, 044003 (2021).
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in