From Signal to Surge: How TNFα/TNFR2 Axis Supercharges NK Cell Activity
Published in Cell & Molecular Biology
Cellular metabolism plays a crucial role in the activation and functional plasticity of immune cells following infection and inflammation, emphasizing the emergence of immunometabolism-focused research in current era 1. Natural Killer (NK) cells are unique innate immune cells that provide the early response during any biological insult without any prior priming 2. Following infection/inflammation, a variety of cytokines are produced. One such cytokine is tumor necrosis factor-alpha (TNFα), which is instrumental in regulating NK cell activity 3. However, TNFα-induced immunomodulatory role in NK cells particularly via metabolism, is not fully understood. TNFα is a pleiotropic cytokine that regulates complex signaling pathways by binding to structurally related but functionally distinct TNFR1 or TNFR2. Signaling pathways through TNFR1 and TNFR2 are intertwined and context-dependent, inducing TNFα-mediated cell activation, proliferation, and cell survival/death 4,5. Given the role of TNFα as a metabolic messenger 6, characterizing the effect of TNFα in the context of respective receptor in NK cell metabolism is critical to exploit TNFαsignal in therapeutics.
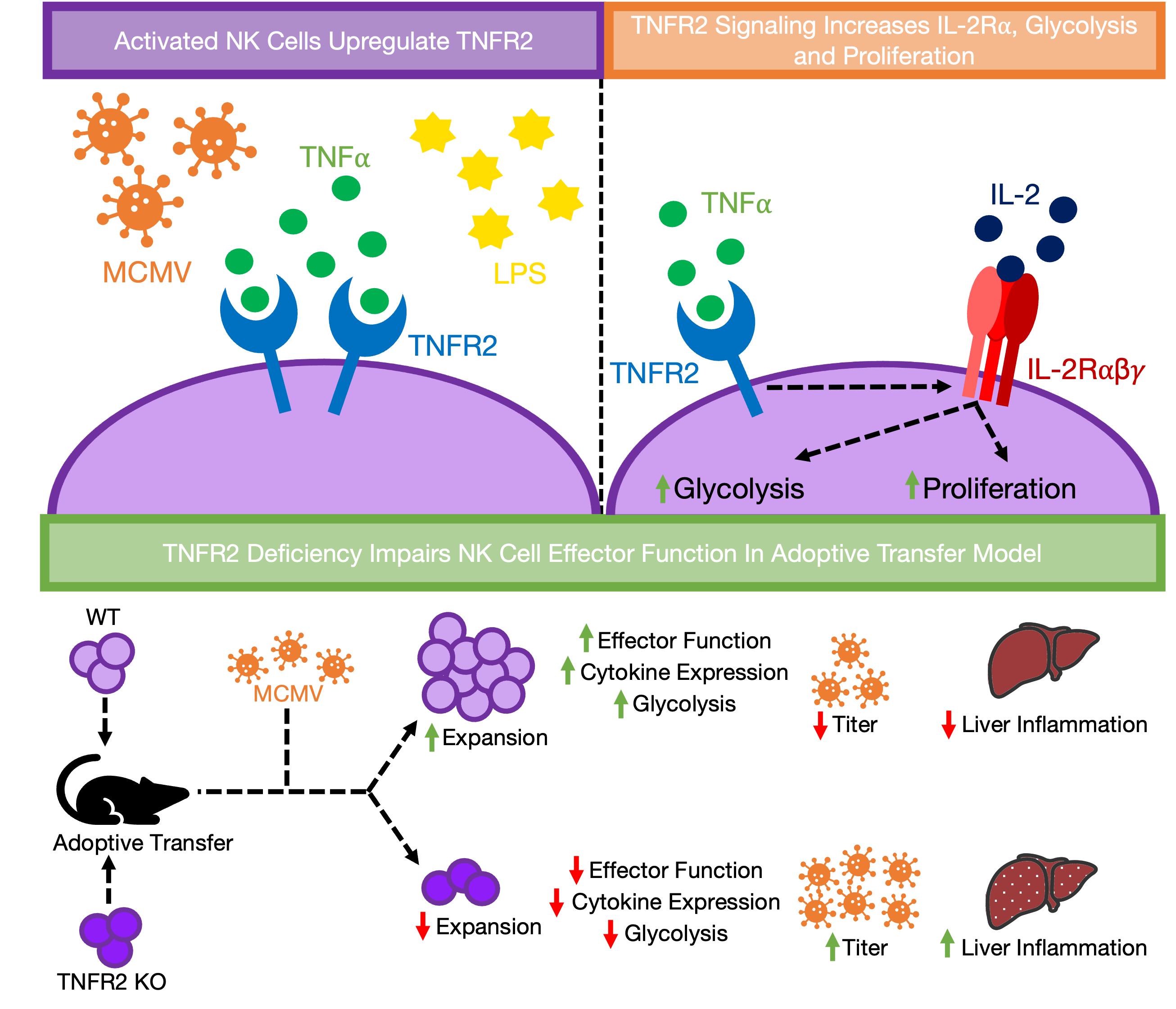
We first investigated the expression of two TNF receptors on NK cells following murine cytomegalovirus (MCMV) infection and LPS-induced inflammation. Our findings provided the hint that the elevated expression of TNFR2, and to a lesser extent that of TNFR1, is a phenotypic signature of activated NK cells. Furthermore, we observed that signaling through IL-18 can induce TNFR2 expression on NK cells. Notably, the TNFR2 induction is largely dependent on the IL-18-MyD88 pathway during LPS-induced inflammation.
NK cell activation is known to be accompanied by proliferation along with elevated metabolic demand, prerequisites for dividing cells 7,8. Accordingly, we assessed the impact of TNFα on NK cell proliferation and metabolic activity. Our ex vivo data revealed that TNFα signaling enhances NK cell proliferation by increasing the responsiveness of NK cells to low doses of IL-2, achieved through the upregulation of the IL-2 receptor alpha chain (IL‐2Rα, CD25). Consistently, the enhanced proliferation correlated with elevated expression of nutrient transporters and metabolic shifting towards glycolysis, leading to enhanced NK cell effector functions. Additionally, signaling through IL-18 furtheramplifies TNFα-induced activation of NK cells by increasing their responsiveness to TNFα through the higher expression of TNFR2 on the cell surface. Importantly, we observed that autocrine TNFα signaling is critical since blocking intrinsic signaling resulted in reduced proliferation, expression of nutrient transporters, and glycolytic metabolism of NK cells.
To further dissect the TNFα-induced activation of NK cells in regard to the respective receptor, we employed mouse models deficient in either TNFR1 or TNFR2. Interestingly, neither TNFR1 nor TNFR2 deficiencies impacts thematuration and the expression of other counterpart receptor of NK cells at the steady-state. Our ex vivo results highlighted the critical role of TNFR2 signaling as NK cells from TNFR2 KO mice displayed significantly decreased proliferation and CD25 expression compared to NK cells from wild-type and TNFR1 KO mice in response to IL-2/TNFα stimulation. In accordance with reduced proliferation, NK cells from TNFR2 KO mice also displayed a decrease in metabolic activity, as evidenced by lower expression of nutrient transporters and a decline in cellular glycolysis..
Finally, we sought to comprehensively characterize the role of TNFR2 signaling in NK cells during in vivo MCMV model. Our transcriptomic analysis revealed that multiple biological processes are compromised in TNFR2-deficient NK cells, including pathways involved in cellular proliferation and metabolism. Notably, genes involved in glycolytic metabolism appeared underrepresented in TNFR2-deficient NK cells during acute viral infection. These results were further validated with a significant decreased Ly49H-induced proliferation and reduced metabolic activity of NK cells following MCMV infection. Importantly, when NK cells from TNFR2 KO mice were adoptively transferred, they provided inferior protection compared to those from wild-type mice, resulting in higher viral burdens and increased liver inflammation during MCMV infection. These results underscore that signaling through TNFR2 is indispensable for NK cell expansion required to clear the virus since extensive proliferation is crucial for efficient anti-viral function in immunocompromised mice 9,10. In summary, we pinpoint that the TNFα-TNFR2 signaling is a major pathway through which NK cells skews the metabolic balance toward aerobic glycolysis, leading to enhanced proliferation and effector function, as depicted in figure.
Recently, NK cells are being exploited for adoptive immunotherapies, including chimeric antigen receptor (CAR)-NK cells 11. For these therapies to be effective, a substantial ex vivo expansion of NK cells is imperative to obtain sufficient numbers of NK cells for adoptive transfer. Given the crital role of TNFR2 signaling in NK cell extensive proliferation and glycolytic metabolism, investigating the TNFα-TNFR2 axis during ex vivo NK cell expansion could be an intriguing avenue for adoptive immunotherapy.
You can access our publication here: https://www.nature.com/articles/s41423-023-01071-4
References:
1 O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nature Reviews Immunology 19, 282-290 (2019).
2 Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nature immunology 9, 503-510 (2008).
3 Biron, C. A. & Tarrio, M. L. Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Medical microbiology and immunology 204, 345-354 (2015).
4 Brenner, D., Blaser, H. & Mak, T. W. Regulation of tumour necrosis factor signalling: live or let die. Nature Reviews Immunology 15, 362-374 (2015).
5 Ward-Kavanagh, L. K., Lin, W. W., Šedý, J. R. & Ware, C. F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 44, 1005-1019 (2016).
6 Sethi, J. K. & Hotamisligil, G. S. Metabolic Messengers: tumour necrosis factor. Nature metabolism 3, 1302-1312 (2021).
7 Khan, A. U. H. et al. Expression of nutrient transporters on NK cells during murine cytomegalovirus infection is MyD88-dependent. Frontiers in immunology 12 (2021).
8 Almutairi, S. M. et al. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid–induced mTORC1 activation in natural killer cells. Journal of Biological Chemistry 294, 4644-4655 (2019).
9 Adams, N. M. et al. Cytomegalovirus infection drives avidity selection of natural killer cells. Immunity 50, 1381-1390. e1385 (2019).
10 Grassmann, S. et al. Distinct surface expression of activating receptor Ly49H drives differential expansion of NK cell clones upon murine cytomegalovirus infection. Immunity 50, 1391-1400. e1394 (2019).
11 McComb, S. & Lee, S.-H. Current Advances and Hurdles in Chimeric Antigen Receptor Technology. Cancers12, 3329 (2020).
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in