Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth
Published in Cancer
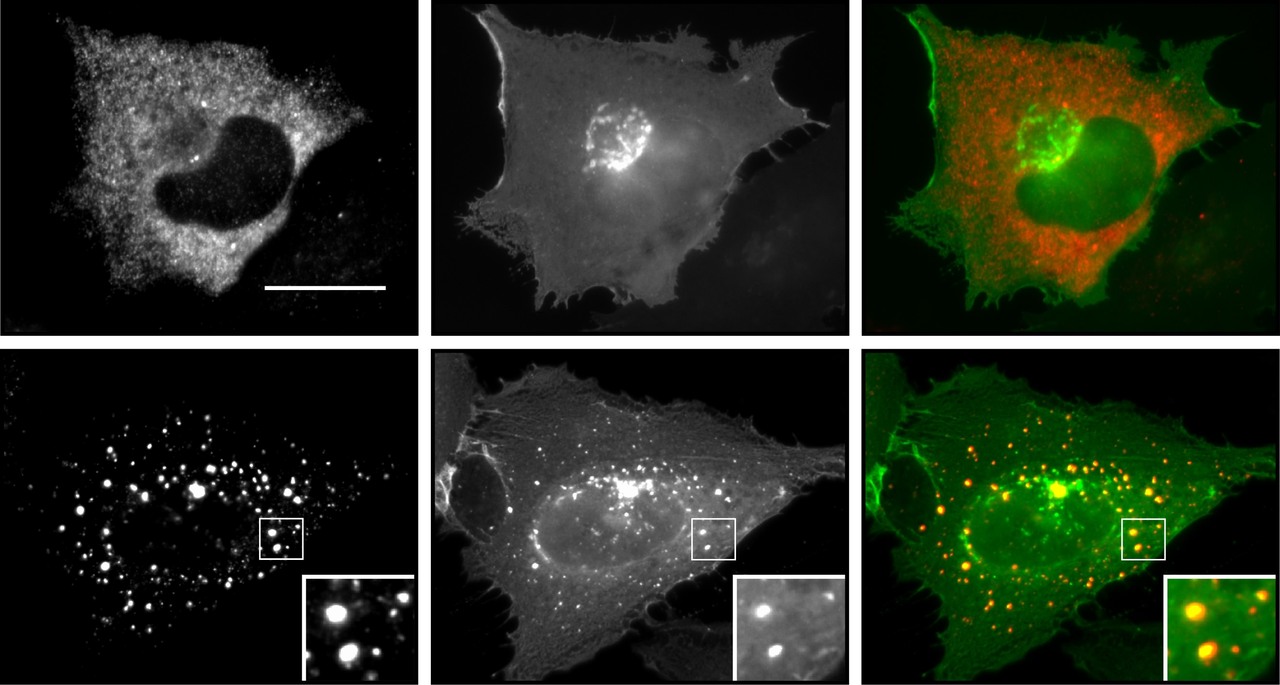
The presented study is part of a more broadly designed research topic characterizing the two homologous negative Wnt pathway regulators axin and conductin/axin2, with the ultimate goal to discover new options for targeted colorectal cancer therapy. The Wnt/β-catenin signaling pathway is hyperactive in more than 90% of colorectal cancers, and promotes tumor growth via the expression of pro-proliferative β-catenin target genes1. Axin and conductin function as scaffold proteins that mediate the assembly of a multiprotein β-catenin destruction complex, thereby inducing β-catenin degradation and silencing the pathway2. The two homologs axin and conductin are well conserved and share a highly similar architecture of protein interaction domains, which mediate binding to destruction complex components. However, we noticed that conductin is less active in degrading β-catenin when directly compared to axin3. Moreover, axin and conductin show strikingly different patterns of cellular distribution. While axin accumulates in densely packed, spherical polymers, which were recently shown to represent biomolecular condensates forming via liquid-liquid phase separation4, 5, conductin appears diffusely distributed throughout the cytosol. Since the importance of axin polymerization for β-catenin degradation was known6, we hypothesized that enforced polymerization of conductin will promote β-catenin degradation. Thus, enforcing conductin polymerization into condensates may offer a chance to inhibit Wnt signaling in colorectal cancer. In addition, conductin is interesting as target for cancer therapy for another reason. Conductin is a β-catenin target gene itself, and, therefore, highly expressed in colorectal cancers because of the hyperactive Wnt/β-catenin signaling pathway7. Although these high expression levels of the negative pathway regulator do not suffice to prevent cancer growth, they may turn out an Achilles’ heel of colorectal cancer, as conductin-based therapies would allow to target cancer cells with high conductin levels but spare healthy cells with lower conductin levels, reducing side effects. That is why we got interested in the differences between the two homologues scaffold proteins, and started to investigate why conductin does not polymerize, whether conductin polymerization can be enforced, and whether this allows regulating Wnt/β-catenin signaling activity.
In our previous study, we discovered an aggregating protein sequence in the regulator of G-protein signaling (RGS) domain of conductin, which is not conserved in axin8. Nano-aggregation of conductin proteins via the N-terminal RGS domain prevents higher order polymerization of the proteins via the C-terminal DIX-domain by a yet unknown mechanism, resulting in the diffuse cellular distribution of conductin. Inhibition of the identified aggregon, both via mutation of key residues or via a small interfering cell-permeable peptide, promoted polymerization of conductin into microscopically visible condensates and enhanced conductin-mediated inhibition of Wnt/β-catenin signaling. Notably, the designed peptide inhibited proliferation of colorectal cancer cells in a conductin-dependent manner, and holds potential for cancer therapy (see figure).
Here, we set out to identify endogenous interactors to the conductin RGS domain that regulate Wnt/β-catenin signaling via the discovered aggregon. Since Gα subunits of trimeric G-proteins are the most prominent interactors of RGS domains, we started the project by testing several Gα proteins for whether their expression enforces polymerization of co-expressed conductin, and detected Gαi2 as positive candidate. The follow-up analysis revealed that Gαi2 directly interacts with the conductin RGS domain, reduces RGS-RGS aggregation by sterically interfering with the aggregon, and induces polymerization of conductin into condensates. Gαi2-enforced condensation of conductin, in turn, promoted β-catenin degradation and inhibited Wnt signaling. Consistently, we characterized Gαi2 as novel negative regulator of the Wnt/β-catenin signaling pathway using gain-of-function and loss-of-function experiments in several cell lines including colorectal cancer cells. During analysis of publicly available data on Gαi2 in colorectal cancer patients, we got excited to see that the mRNA expression of Gαi2 negatively correlates with the expression of β-catenin target genes, and that colorectal cancers exhibit reduced Gαi2 mRNA expression compared to healthy tissue. Moreover, occurring Gαi2 cancer mutants were less active in promoting condensation of conductin and inhibited Wnt signaling less efficiently than WT Gαi2. Notably, colon cancer patients with such Gαi2 mutations (1%) or with reduced copy numbers of the Gαi2 gene (5%) showed a markedly lower 3-year survival rate compared to patients without Gαi2 aberrations. These data are consistent with Gαi2-mediated inhibition of Wnt/β-catenin signaling in colorectal tissue, which some cancers evade via inactivating Gαi2 mutations or via reduced Gαi2 expression, thereby becoming more aggressive. Obviously, inhibition of Wnt signaling via the discovered Gαi2-conductin axis may be exploitable for targeted cancer therapy, if one could activate Gαi2, and, thus, we started searching for Gαi2-activating substances. Gαi2 is activated via the classical activation cascade of trimeric G-proteins by G-protein coupled receptors; in case of Gαi2 e.g. α2-adrenoceptors. Fortunately, α2-adrenoceptors have been extensively studied as pharmacological targets of antihypertensive drugs, and there are several FDA-approved substances available that activate these receptors, such as guanabenz. In our experiments, guanabenz treatment enforced polymerization of conductin into condensates, and inhibited Wnt signaling as well as proliferation of colorectal cancer cells in a conductin-dependent fashion (see figure). Importantly, oral guanabenz application via the drinking water inhibited growth of Wnt signaling-driven intestinal tumors in two mouse models. The required guanabenz amount for tumor treatment in mice translates to a human equivalent dose that is about 4 times less than the maximal routinely used dose for treatment of hypertension patients. We, therefore, think that guanabenz treatment offers a real chance for targeted colorectal cancer therapy. In summary, what started out as a comparison of two highly related scaffold proteins, axin and conductin, in the end, revealed a novel layer of Wnt pathway regulation via the enforced polymerization of conductin by Gαi2, and indicated novel options for cancer treatment.

- Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330-337 (2012).
- Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harbor perspectives in biology 5, a007898 (2013).
- Bernkopf DB, Hadjihannas MV, Behrens J. Negative-feedback regulation of the Wnt pathway by conductin/axin2 involves insensitivity to upstream signalling. J Cell Sci 128, 33-39 (2015).
- Fagotto F, et al. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145, 741-756 (1999).
- Nong J, Kang K, Shi Q, Zhu X, Tao Q, Chen YG. Phase separation of Axin organizes the beta-catenin destruction complex. J Cell Biol 220, (2021).
- Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc Natl Acad Sci U S A 108, 1937-1942 (2011).
- Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22, 1184-1193 (2002).
- Bernkopf DB, Bruckner M, Hadjihannas MV, Behrens J. An aggregon in conductin/axin2 regulates Wnt/beta-catenin signaling and holds potential for cancer therapy. Nat Commun 10, 4251 (2019).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in