General synthesis of high-entropy single-atom nanocages for electrosynthesis of ammonia from nitrate
Published in Chemistry, Ecology & Evolution, and Materials

The nitrogen cycle is a crucial biogeochemical process on the Earth, but the excessive presence of nitrogen compounds in water is a significant ecological threat. Nitrate (NO3-), a major nitrogen pollutant, is commonly found in groundwater, rivers, and lakes, underscoring the urgent need to restore balance to the nitrogen cycle and reduce nitrate contamination. Traditional nitrate removal methods, such as using anaerobic bacteria or physicochemical techniques, often require significant capital investment and maintenance. In contrast, electrochemical reduction presents a promising, environmentally friendly alternative, offering efficient nitrate removal and ammonia synthesis with a small installation footprint and low operational costs. This technology, driven by electricity, can utilize renewable energy sources like wind and solar power, thus reducing overall energy consumption. However, the electrocatalytic reduction of nitrate to ammonia is a complex process involving the transfer of 9 protons and 8 electrons, which requires high-performance catalysts and precise control over reaction conditions, such as electrolyte concentration and potential. Continued research into catalyst development and process optimization is essential to enhance sustainable nitrogen management practices.
To this critical challenge, this work provides a proof-of-concept synthesis of high-entropy single-atom nanocages (HESA NCs) through a two-step process. This method begins with cyano-reaction and 3D polymerization in an aqueous solution under ambient conditions, resulting in cyanogel-based nanocubes with a medium-entropy single-atom lattice (MESA nanocubes) that serve as templates for subsequent etching. Unlike high-entropy alloy (HEA) nanoparticles (NPs) where metal atoms are connected by metallic bonds, in MESA nanocubes, metal atoms or ions are isolated and covalently coordinated with nitrogen or carbon within the lattice (Figure 1). As a result, despite both HEAs and MESA nanocubes exhibiting face-centered cubic (fcc) lattices of metal atoms with high or medium entropy, their differing bond structures lead to distinct behaviors during chemical etching. Specifically, for HEA NPs, random surface etching is thermodynamically favorable, producing nanoparticles with irregular surfaces (iNPs) due to arbitrary lattice vacancies. In contrast, spatially selective etching of MESA nanocubes can be controlled to generate lattice vacancies selectively, removing the internal region of the nanocubes and forming HESA NCs.
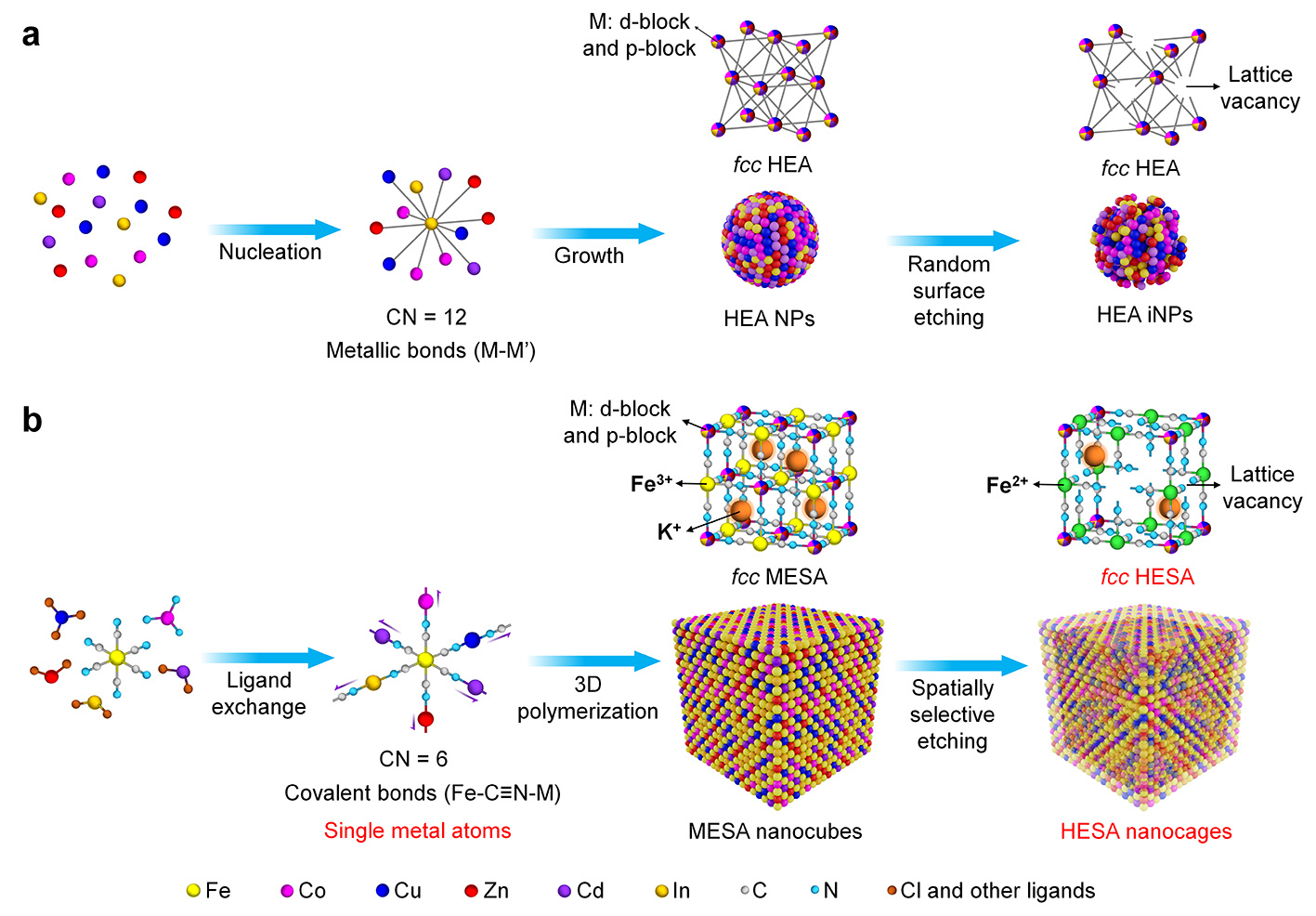
Figure 1. Schematic illustration of the HEA iNPs and HESA NCs generation. a) Colloidal synthesis of HEA iNPs starts with homogeneous nucleation of metal atoms connected by metallic bonds, followed by the growth of nuclei into NPs. After an oxidative etching process, the HEA NPs are supposed to deliver an irregular shape due to the random etching of surface atoms. b) In the case of HESA NCs, the MESA nanocubes are firstly formed via ligand exchange and cyano polymerization, followed by selective etching of the internal space, generating HESA NCs.
The key to the synthetic strategy is the introduction of heterogeneous distributions of metallic elements into the lattice of the cyanogel nanocubes in which metal ions coordinate with −N≡C−Fe(III) and −N≡C−Fe(II) in the core and shell, respectively. Upon reacting with ammonia, the bonding between metal ions and −N≡C−Fe(III) can be readily broken via a ligand exchange reaction, resulting in a spatially selective etching process for the transformation of MESA nanocubes to HESA NCs with increased mixed configurational entropy.
To trace and quantify the changes of MESA nanocubes in terms of morphology, composition, chemical structure, and lattice parameters during etching, we designed a series of ex-situ experiments to simulate the time-dependent in-situ characterizations. Using this method, we have successfully captured intermediate samples during the fast etching process. Initial reaction with ammonia resulted in the generation of a yolk@shell structure likely due to the contact of outmost lattice points of Mn+ coordinated with −N≡C−Fe(III) in the core region with ammonia. As the reaction continues, the internal region of the nanocube became visibly void, generating a double shell structure. This special structure might be caused by the redox reaction between terminal [Fe(III)(CN)6] and ammonia, resulting in newly generated M−N≡C−Fe(II) with a higher etching resistibility inside the nanocube. As the etching proceeded, the inner shell was completely removed while the outer shell with a larger thickness and high internal surface roughness was observed, which was considered as the involvement of dissolution and recrystallization processes of Fe(II)/Fe(III) lattice points. Finally, a hollow nanobox with flat internal and external surfaces was obtained with increased extent of etching. Further etching of the nanobox increased the porosity of the walls, producing a nanocage with observable cavities on the walls. This result indicates the existence of M−N≡C−Fe(III) on the walls of the nanobox which could be etched away after sufficient etching time or under a robust etching condition.
Further, we utilized our Fe-HESA NCs as a model high-entropy catalytic platform for nitrate reduction reaction (NO3RR) to illuminate their structure-property-performance relationships and high-entropy effects via benchmarking with the Fe-Co NCs counterparts. Our Fe-HESA NCs catalyst demonstrated high selectivity for producing NH3 from NO3RR, with an outstanding Faradaic efficiency (FE) and a high yield rate (YR) of NH3. Additionally, Fe-HESA NCs showed catalytic stability across 20 consecutive electrolysis cycles.
To investigate the reaction process and mechanism of NO3RR on the Fe-HESA NCs, we used an operando synchrotron-radiation Fourier transform infrared spectroscopy (SR-FTIR) to unravel the adsorbates on the catalyst surface during the reaction. The fast increase of Had signals indicates the enhanced water dissociation at higher voltage, which is beneficial to the hydrogenation process of NO3RR for the generation of NH3. The entropy-induced symmetry reduction of single-atom lattice combined with the enhanced water dissociation and thus the hydrogenation process of NO3RR leads to high activity, selectivity, and durability of the catalyst, delivering both high FE and YR of NH3 over continuous 20 cycles of electrolysis. The findings of this study provide an insightful guideline for fabricating scalable yet controllable hollow nanostructures with single-atom lattice and high entropy for sustainable electrocatalytic reactions.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in