Parkinson’s disease (PD) is the second most common neurodegenerative disorder. In most cases it occurs sporadically, with the risk of developing PD likely resulting from the complex interaction between many genetic and environmental risk factors. However, in some instances PD occurs due to changes in a single gene (monogenic).
Disease-causing genetic variants in about 20 different genes have been reported in monogenic PD. The likelihood of carrying at least one of these disease-causing variants increases in the presence of a family history of PD, and with earlier age at disease onset. However, even cases of early-onset or familial PD have a relatively low rate of positive genetic findings, indicating that additional monogenic forms of PD are yet to be uncovered and/or that some families have more complex inheritance. As with other adult-onset genetic disorders, identifying and validating candidate disease-causing variants in PD are complicated by the fact that DNA is often unavailable from family members across generations, which is necessary to establish a causal relationship between the genetic variation and the disease phenotype.
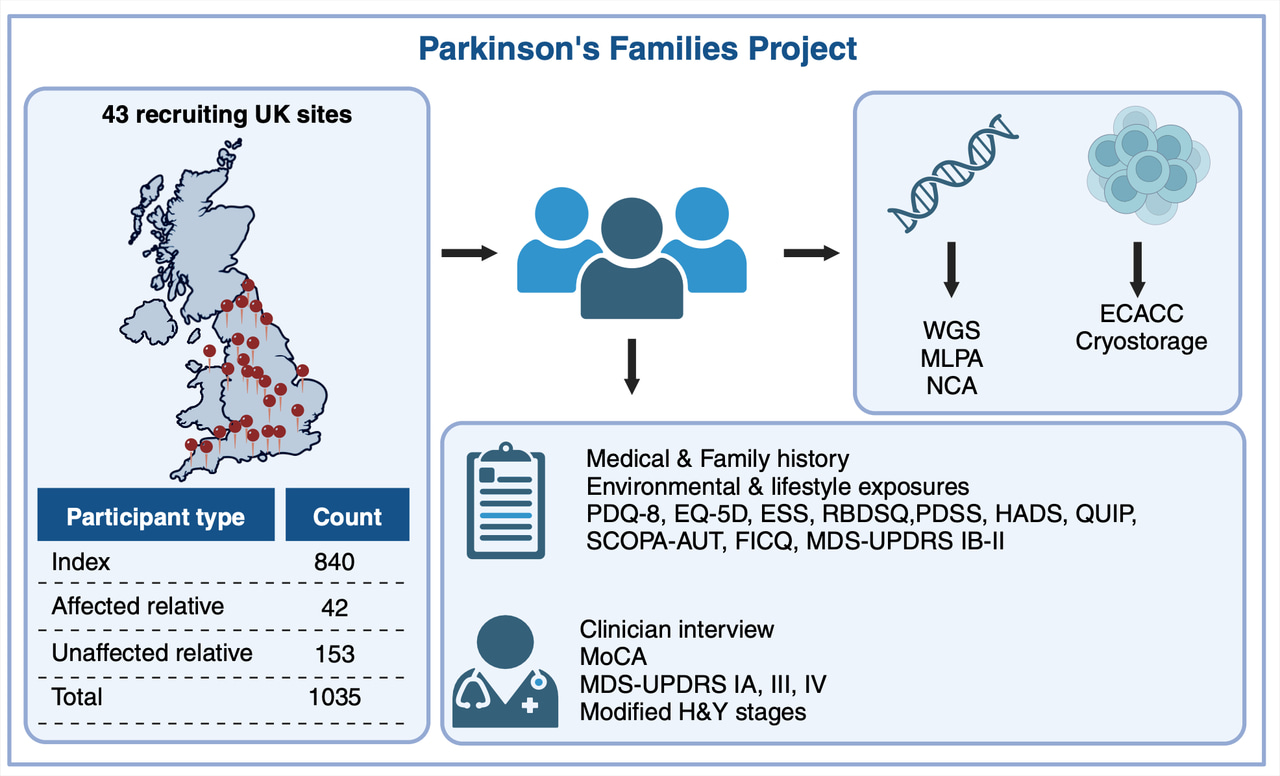
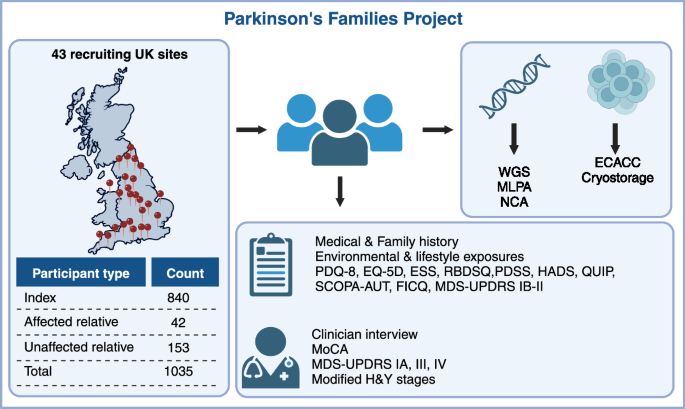
The Parkinson’s Families Project (PFP) was therefore set up with the aim of building a PD cohort theoretically enriched in monogenic forms of PD to facilitate genetic discovery. We have recruited 718 families with early onset and/or familial PD, including affected and unaffected family members. We collected detailed demographic, lifestyle, environmental, pharmacological and clinical data, using standardised scales and questionnaires, as well as blood samples for DNA extraction for genetic analysis.
What were the main findings? We have found disease-causing variants in PD-related genes in 9.6% of families, with the rate of a positive finding increasing to 28.1% in families with age at disease onset ≤35 years old, reflecting the increased likelihood of monogenic PD among individuals with early onset disease. Most families carried pathogenic variants in LRRK2 (4.2%) and PRKN (3.6%), but pathogenic variants were also found in SNCA, ATXN2, VCP, PINK1, PNPLA6, PLA2G6, SPG7, GCH1, and RAB32 genes. Some of these genes are not typically associated with PD, although levodopa-responsive parkinsonism has been previously described for all of them. In particular, ATXN2 expansions typically associated with ataxia were the third most common cause of familial PD in this cohort. This suggests that genetic testing of early onset and/or familial PD should include a comprehensive panel of genes outside the more typical PD-related genes.
An additional 10.2% of families carried a genetic variant in GBA1, thus confirming GBA1 as the single most important genetic risk factor for PD. Families with GBA1 variants often presented with a pattern akin to autosomal dominant inheritance. We confirmed that GBA1 carriers performed worse on cognitive testing and that the effect of GBA1 on cognition was dependent on the severity of the variant, as reported by others.
What are the next steps? Most early-onset and familial PD cases do not have a known genetic cause, suggesting that additional causative or contributing genetic factors are yet to be uncovered. Families with a seemingly strong genetic component that remain without a molecular diagnosis present an opportunity to uncover novel causative or high-risk conferring genetic variants and will be the focus of the next phase of the analysis, including newer sequencing technologies. The Parkinson’s Families Project will continue to recruit from currently participating and new families until 2030.
Why is it important? Genetic forms of PD can be clinically indistinguishable from the most common sporadic form of the disease, but their recognition is important for several reasons. First, there is overlap between pathophysiological mechanisms of sporadic and genetic forms of PD, which means that studying monogenic PD can elucidate yet unknown disease mechanisms. Second, drugs targeting molecular defects present in specific forms of monogenic PD (e.g. LRRK2, GBA1) are already under development. It is reasonable to expect that, as we expand our knowledge of monogenic PD, novel targets for drug development will be identified that could also be beneficial in sporadic PD. Lastly, carrying a disease-causing genetic variant has implications for other family members and family planning decisions, a particularly relevant aspect in monogenic PD, which often affects individuals of reproductive age. The more we know about which genes cause PD, the better we can support informed life decisions for affected individuals.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in