Growing meat on autoclaved vegetables
Published in Bioengineering & Biotechnology, Sustainability, and Agricultural & Food Science

Never Take Meat for Granted
A healthy diet contains meat. But meat doesn’t come easy. It’s not just the neatly packaged cuts on supermarket shelves, which we can always buy for granted. Behind the juicy cuts means acres of farmland, crowded herds, piles of feed, tons of water and waste, and countless hours of human labor. That is why researchers, including us, are working on “cultured meat”, a sustainable alternative to “growing” meat in the lab, hopefully without animal farming and killing [1].
A Bumpy Road with Plants
Our first attempt at cultured meat was to use porous micro-carrier scaffolds—mini spongy spheres made of gelatin, to massively produce animal muscle and fat cells [2]. However, gelatin is animal-derived, and we felt an urge to upgrade to more sustainable materials, like plants.
Plants, in their decellularized format, have already been proposed by scientists as cell culture materials [3-5]. So I started decellularizing plants, with a lot of patience, of course. After spending half a week soaking plants in surfactants and washing out unwanted plant cells, I finally obtained a delicate cellulose skeleton. Sadly, when I tried to grow muscle cells on decellularized plants (DP), cells hardly survived. They wouldn’t adhere to the cellulosic matrices lacking cell-affinitive proteins. So I went on testing many reported ways of surface modification [4,6], hoping to make cells happy. However, 3 months had passed, and my experiments still went nowhere.
A Flash of Dumb Luck
“Say bye to plants”, I told myself. But just before throwing the leaves and potatoes in the trash, l decided to give them a last chance, while doing it differently, just for fun. So the remaining, undecellularized plants were tossed into an autoclave (a machine to sterilize biomaterials) and carelessly seeded with cells. Surprisingly, when I came back two days later, cells seemed to thrive on undecellularized plants!
It turned out that decellularization was not necessary. The trick? A quick bath in the autoclave steam! I was so relieved. This plant autoclaving method (just like cooking) is simple and fast, and way more effective than my previous complicated trials.
A Grocery Store Adventure
The next step was to find the right plants, with muscle- and fat-like surface textures. This is important for meat-engineering, because tissue-mimicking micro-structures will give cells the instructions to properly organize and develop into something meat-like.
I thus became a regular at the local supermarket, lugging bags of vegetables and fruits back to the lab. I even went online hunting exotic plants like mini lotus sprouts and Zingiber striolatum Diels (a red bulb that looks like a ginger-onion hybrid). Being someone who never bothers to cook, I had to cook my plant failures. Believe it or not, I’ve tasted some weird stuff I’ll never try for a second time.
The Secret of Autoclaving
Luckily, this didn’t last for long. I found that Chinese chives and Shiitake mushrooms were excellent for hosting muscle cells, while loofah worked well for fat cells. Not only did the plants support cell growth, but they helped cells mature into layers of muscle and fat tissues.
With chemical analysis, we uncovered the magic behind autoclaving. When plants are autoclaved, they preserve cell-friendly functional groups (like amides), which are otherwise missing from surfactant-treated DP. That is why mammalian cells proliferate so nicely on them. Autoclaved plants also keep their natural micro-patterns, which help cells align in the right order. And, as a bonus, autoclaving softens plants to match the stiffness of real muscle and fat, which is also important for cell differentiation.
In short, autoclaving transforms ordinary plants into biomimetic, cost-effective scaffolds for cultured meat (Figure 1), without expensive materials or complicated procedures.
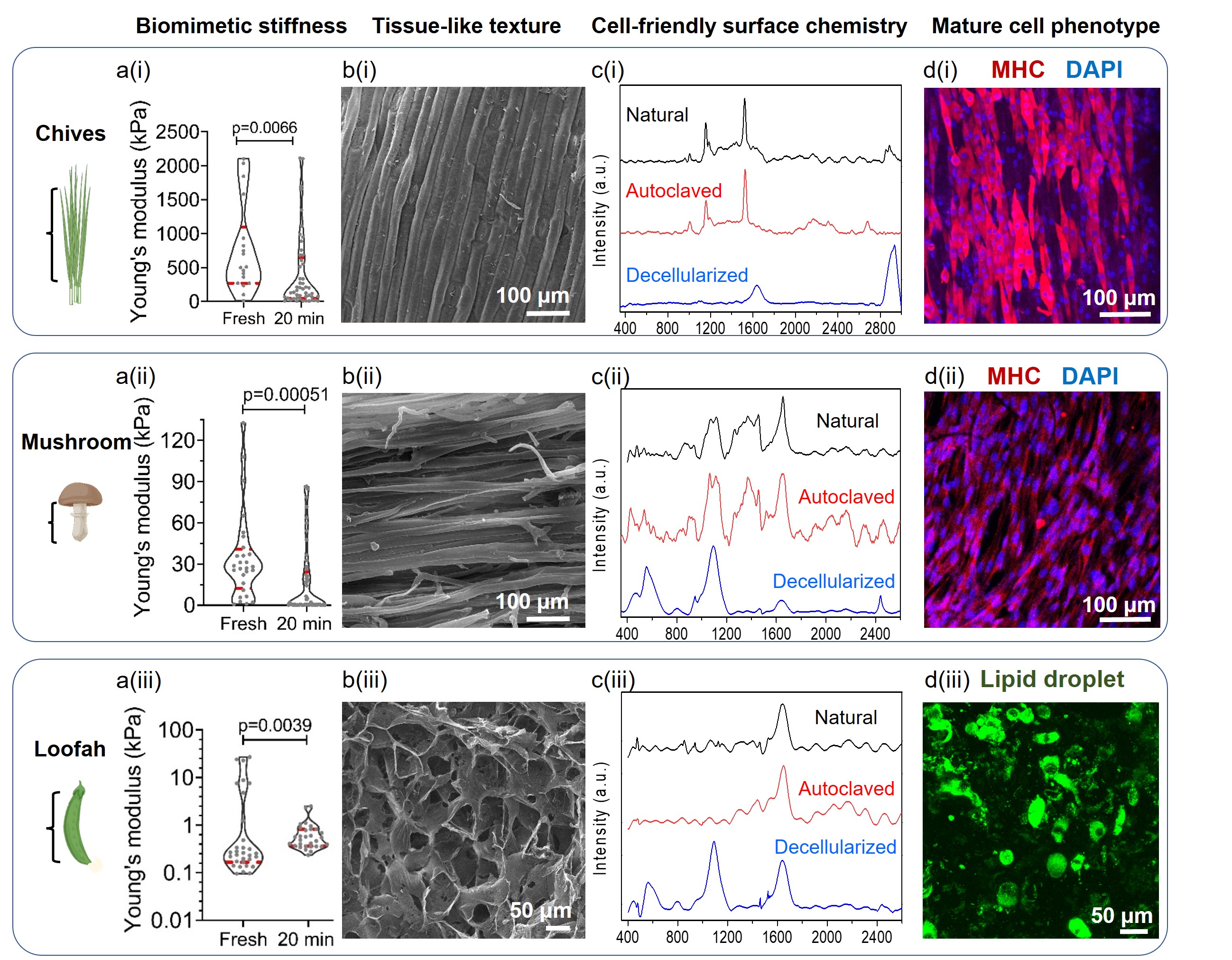
Figure 1. Autoclaved vegetables with biomimetic stiffness and micro-patterns promote the proliferation and differentiation of muscle and fat cells. (a) Young’s modulus of fresh and autoclaved Chinese chive leaves, Shiitake mushroom stems, and loofah slices. Magenta lines represent the quartiles. (b) SEM images showing surface morphology of Chinese chive leaves, Shiitake mushroom stems, and loofah slices. (c) Raman spectra of Chinese chives, Shiitake mushroom and loofah under different treatments. (d) Staining of terminal differentiation marker MHC in muscle cells on Chinese chives and mushroom scaffolds, and lipid droplets in differentiated fat cells on loofah scaffolds.
Unconditional Support from the Team
While it might sound like a small personal story, behind me is a loving team. I got incredible support from my postdoc supervisor, Professor Yanan Du at Tsinghua University, who inspired me to dive into the world of cultured meat, and gave me the freedom to mess things up. I also benefit from a very collaborative and educative culture in the lab. My lab mates, mostly young master's and PhD students, are all great teachers. Everyone has specific sets of expertise and is always ready to help, whether it’s troubleshooting failed experiments or teaching me new skills. We even have a fun tradition: everyone creates a short film (something like research TikToks) showcasing their proudest experiments and research moments. Over the years, our lab has built a library of research tips and tricks, which is a great treasure for all members. Now that I become an independent researcher at Beijing Institute of Technology, I’ve realized that it is a great way to cultivate young people, and to build a family where everybody contributes and grows together.
No Effort and Mistakes are Wasted
And, don’t forget the fails and pains! Repeated experiments, long nights in the lab, the summer heat and winter blast—it’s all part of the journey, which is probably true for all researchers. Months of hard work could be ruined, by a tiny mistake or mishap. But sometimes you have to get things wrong, before you can get them right. When planned experiments turned unplanned, serendipity may sneak in. And probably, the best discoveries happen when you least expect them.
Plants: Not Just for Salad
Finally, plants mean more than just food. With a little bit of autoclaving, plants can become tasty scaffolds to grow lab-made meat. Autoclaved plants possess cell-friendly surface chemistry and intricate textures that no human engineer could replicate. They might even see applications beyond meat—perhaps in tissue regeneration or soft robotics. It all depends on how we look at the ordinary yet amazing world around us.
References
- Post, M. J. et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat Food 1, 403–415 (2020).
- Liu, Y. et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials 287, 121615 (2022).
- Modulevsky, D. J., Lefebvre, C., Haase, K., Al-Rekabi, Z. & Pelling, A. E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS One 9, e97835- (2014).
- Fontana, G. et al. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv Healthc Mater 6, 1601225 (2017).
- Gershlak, J. R. et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 125, 13–22 (2017).
- H, A., Mohan, C. C., P.S, U., Krishnan, A. G. & Nair, M. B. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Materials Science and Engineering: C 119, 111500 (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in