Gut colonisation with multidrug-resistant Klebsiella pneumoniae worsens Pseudomonas aeruginosa lung infection
Published in Microbiology
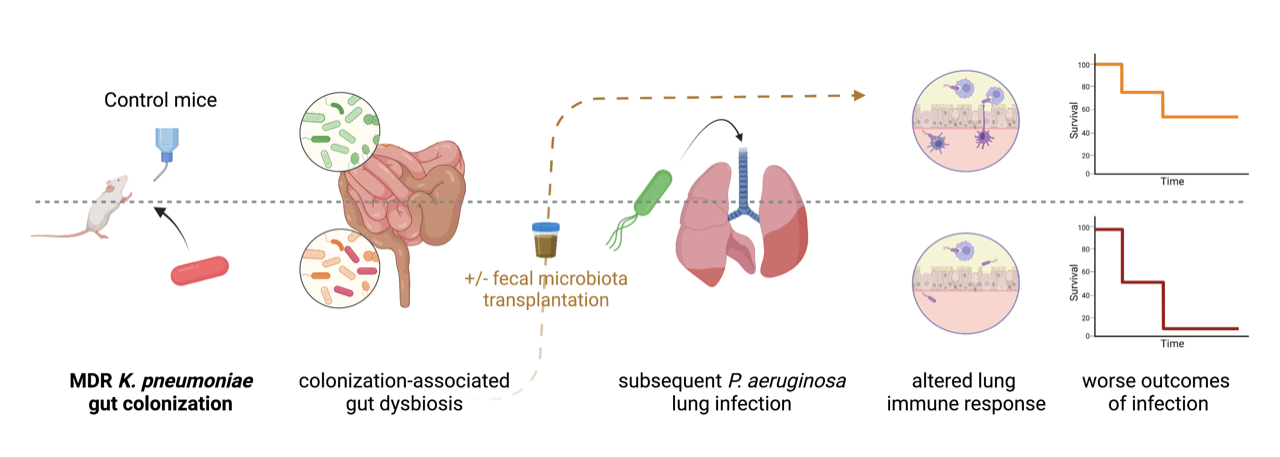
Despite recognizing the worldwide spread of carbapenemase-producing Enterobacterales (CPE) bacteria as a looming healthcare crisis from an infection control standpoint, little is known about the impact of being colonized by CPE on the gut-lung axis. This is a surprising gap in knowledge given that gut colonization by CPE involves an imbalance in the gut microbiota1, or dysbiosis, that provides an ecological “niche” for colonizing bacteria and that gut dysbiosis from various causes is known to alter immune responses2.
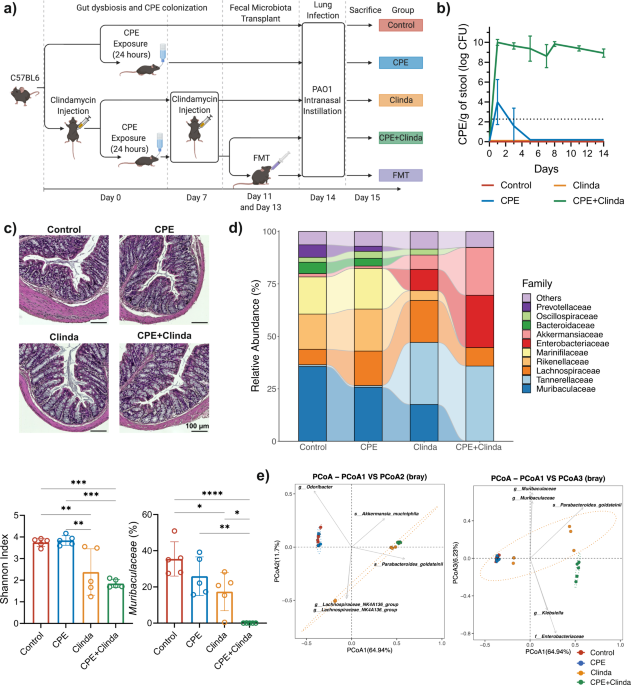
We sought to determine whether gut colonization with carbapenemase-producing Klebsiella pneumoniae could impact the outcomes of lung infection in a murine model. Mice were colonized by CPE for two weeks, following exposure to NDM-producing K. pneumoniae in drinking water and injection of an antibiotic (clindamycin) to overcome colonization resistance of the healthy gut microbiota. Then, these mice were subsequently infected intranasally by Pseudomonas aeruginosa, a Gram-negative bacterium frequently responsible for healthcare-associated pneumonia.
Gut colonization with CPE was associated with a specific gut dysbiosis, different from antibiotic-induced dysbiosis, depressed cellular lung immune response to P. aeruginosa infection, and worse outcomes. Faecal microbiota transplantation (FMT) restored the lung immune response and outcomes of lung infection without decolonizing the gut, demonstrating the involvement of gut dysbiosis (rather than the presence of K. pneumoniae) in altering the gut-lung axis. Likewise, restoration of outcomes by administration of short-chain fatty acids (SCFA), previously reported to protect against lung infection3, provides a possible link between gut dysbiosis and lung immunity in our model. The main finding is that gut colonization by CPE, specifically carbapenemase-producing K. pneumoniae in our model, may not be the “silent” phenomenon it is usually considered to be, and this associated gut dysbiosis may impede responses to infection with worse outcomes.
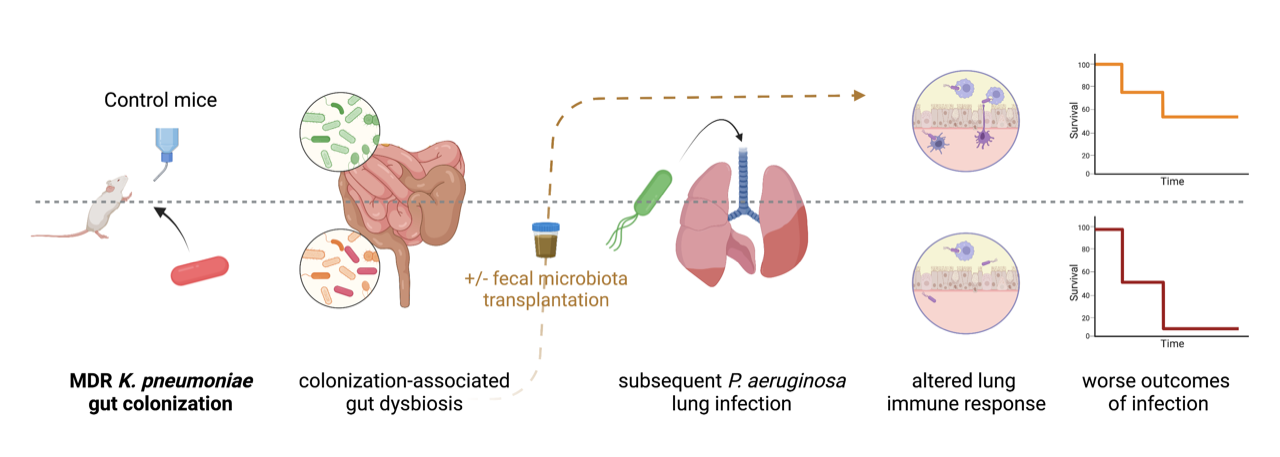
Are there data suggesting these findings may extrapolate to real-life patients? While studies in the intensive care unit show an increased death rate in patients colonized with CPE, these colonized patients include those who ultimately develop infections due to CPE, which are challenging to treat due to multidrug resistance and have understandably poorer outcomes4. The most compelling clues are found outside of the ICU. In a study of the gut microbiome composition of 7211 adults in Finland with a 15-year long-term follow-up through access to electronic medical records, an increased abundance of Enterobacterales in the gut was associated with higher all-cause mortality and higher mortality from respiratory causes5. Another study comparing the gut microbiota of hospitalized internal medicine patients colonized or not with CPE showed that CPE-colonized patients had a more pronounced gut dysbiosis than non-colonized patients, despite both groups having been similarly exposed to antibiotics1. Interestingly, twofold more bacteraemia was found in the CPE carriers than in the noncarriers and was not exclusively Enterobacterales1. This is similar to our finding that among the worse outcomes of P. aeruginosa lung infection in mice with CPE colonization-associated gut dysbiosis was increased dissemination of P. aeruginosa to the spleen.
While restricted to K. pneumoniae, our model was not strain/carbapenemase specific since various strains with different carbapenemase resistance mechanisms (NDM-1, OXA-48, KPC) resulted in the same observations. Furthermore, the impact of colonization on the immune response to/outcomes of lung infection does not seem to depend solely on the presence in the gut of K. pneumoniae since FMT restored the control lung infection phenotype without decolonization.
A specific CPE colonization-associated dysbiosis seems to be the key to our findings since a) clindamycin, which was used to provide the ecological niche for gut colonization by CPE, was associated with a different dysbiosis with no impact on P. aeruginosa lung infection outcomes, and b) FMT in CPE colonized mice did restore immune response and outcomes of P. aeruginosa lung infection, without reducing CPE loads (i.e., without ‘decolonizing’ the gut) within the study timeframe. Our results suggest an interplay between the dysbiotic niche provided by antibiotics for K. pneumoniae to colonize the gut in conjunction with the specific dysbiotic imbalance maintained by the presence of K. pneumoniae. Rebalancing the dysbiosis accompanying CPE gut colonization may be sufficient to mitigate the deleterious impact on the immune response to infection without requiring decolonization, which is difficult to achieve. In hospitalized patients colonized by multidrug-resistant bacteria, FMT significantly reduced the amount of bacteraemia despite modest rates of intestinal decolonization6. Future clinical trials should address the impact of FMT on patient outcomes, such as protection against invasive infection7.
The results provided by our murine model question the reality of “asymptomatic” carriage of CPE that may in fact be associated with dysbiosis impacting patient outcomes. Furthermore, “decolonization” may not represent the main issue compared to rebalancing the microbiota through approaches such as faecal microbiota transplantation or rebalancing the immune response through immunomodulatory approaches.
References
1 Korach-Rechtman, H. et al. Intestinal Dysbiosis in Carriers of Carbapenem-Resistant Enterobacteriaceae. mSphere 5 (2020). https://doi.org:10.1128/mSphere.00173-20
2 Wypych, T. P., Wickramasinghe, L. C. & Marsland, B. J. The influence of the microbiome on respiratory health. Nat Immunol 20, 1279-1290 (2019). https://doi.org:10.1038/s41590-019-0451-9
3 Machado, M. G., Sencio, V. & Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: a Closer Look at the Lungs. Infect Immun 89, e0018821 (2021). https://doi.org:10.1128/IAI.00188-21
4 Pilmis, B. et al. Multidrug-resistant Enterobacterales infections in abdominal solid organ transplantation. Clin Microbiol Infect 29, 38-43 (2023). https://doi.org:10.1016/j.cmi.2022.06.005
5 Salosensaari, A. et al. Taxonomic signatures of cause-specific mortality risk in human gut microbiome. Nat Commun 12, 2671 (2021). https://doi.org:10.1038/s41467-021-22962-y
6 Ghani, R. et al. Disease Prevention Not Decolonization: A Model for Fecal Microbiota Transplantation in Patients Colonized With Multidrug-resistant Organisms. Clin Infect Dis 72, 1444-1447 (2021). https://doi.org:10.1093/cid/ciaa948
7 Ghani, R., Mullish, B. H., Davies, F. J. & Marchesi, J. R. How to Adapt an Intestinal Microbiota Transplantation program to reduce the risk of invasive multidrug-resistant infection. Clin Microbiol Infect (2021). https://doi.org:10.1016/j.cmi.2021.11.006
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in