
For many years, our research group has been using neonatal rat ventricular cardiomyocytes (NRVMs) for our in vitro research into ventricular tachyarrhythmias as these cells maintain their functional properties in culture for at least 6 months [1] in contrast to adult mammalian cardiomyocytes. Moreover, using hearts of newborn rats instead of neonatal mice as starting material for the isolation of ventricular cardiomyocytes yields 4 to 5 times more viable cells per heart. In 2012, we became interested in atrial fibrillation and started to work with neonatal rat atrial cardiomyocytes (NRAMs). Using optogenetically modified NRAMs, we were the first to demonstrate the feasibility of terminating reentrant arrhythmias by light [2]. The NRAMs were also used to investigate the role of the acetylcholine-activated inward-rectifying potassium current (IKACh) in the induction, dynamics and termination of atrial fibrillation [3,4].
A major problem associated with the use of NRAMs to study atrial tachyarrhythmias is the low number of these post-mitotic cells that can be obtained from a single heart (~15-fold less than NRVMs), which forced us to sacrifice many rat pups to obtain sufficient cells for our monolayer studies. This led to the idea to establish lines of NRAMs for our research. Based on previous experiments showing the ability of simian virus 40 (SV40) large T antigen (LT) to immortalize murine atrial cardiomyocytes [5,6], we decided to also employ this oncoprotein for our purpose. However, instead of using a constitutively expressed transgene, the expression of LT (and the other early gene products of SV40) was made conditional on the presence of doxycycline to separate in time cell proliferation from cell differentiation as for many cell types continuous LT expression/cell proliferation negatively affects their functional properties. Consistent with this notion, several studies have shown a negative effect of continuous LT expression on the differentiation ability of (ventricular) cardiomyocytes [7,8]. Moreover, to minimize the risk of immortalizing other cardiac cell types present in the NRAM preparations, the expression of the LT was driven by a striated muscle-specific promoter. The choice of a cell type-specific promoter to drive LT expression was also intended to ensure that the NRAMs retain some basic gene regulatory circuits of cardiomyocytes during proliferation since the complete loss of cardiac transcriptional hallmarks would cause cessation of transgene expression. This, in turn, would result in loss of cell division precluding the establishment of lines of conditionally immortalized NRAMs.
Approximately 2 weeks after lentiviral vector-mediated LT delivery into primary NRAMs, colonies of proliferating cells started to appear in cultures fed with doxycycline-containing medium suggesting successful immortalization of the cells. At this stage, we were, however, still afraid to have immortalized cardiac fibroblasts or vascular smooth muscle cells present in our starting material or to have largely/completely erased the epigenetic memory of the NRAMs’ original cell identity, which would preclude the spontaneous cardiomyogenic differentiation of the (conditionally) immortalized cells after doxycycline removal. These fears appeared to be unfounded since immunostaining of the cells derived from individual clones for sarcomeric α-actinin and Ki-67 or cardiac troponin T and LT after 9 days of culture in the absence of doxycycline unambiguously demonstrated the formation of well-structured cardiomyocytes in ~10% of the clones (see Figure). These clones were named iAMs, which stands for immortalized Atrial Myocytes. Extensive characterization studies of the iAM clones showed that the cells lost most cardiomyocyte traits and proliferated at a doubling rate of ~2 days in the absence of doxycycline while after doxycycline removal, the cells stopped dividing and spontaneously differentiated into cardiomyocytes with structural and functional properties superior to the differentiated progeny of all existing cardiomyocyte lines [9].
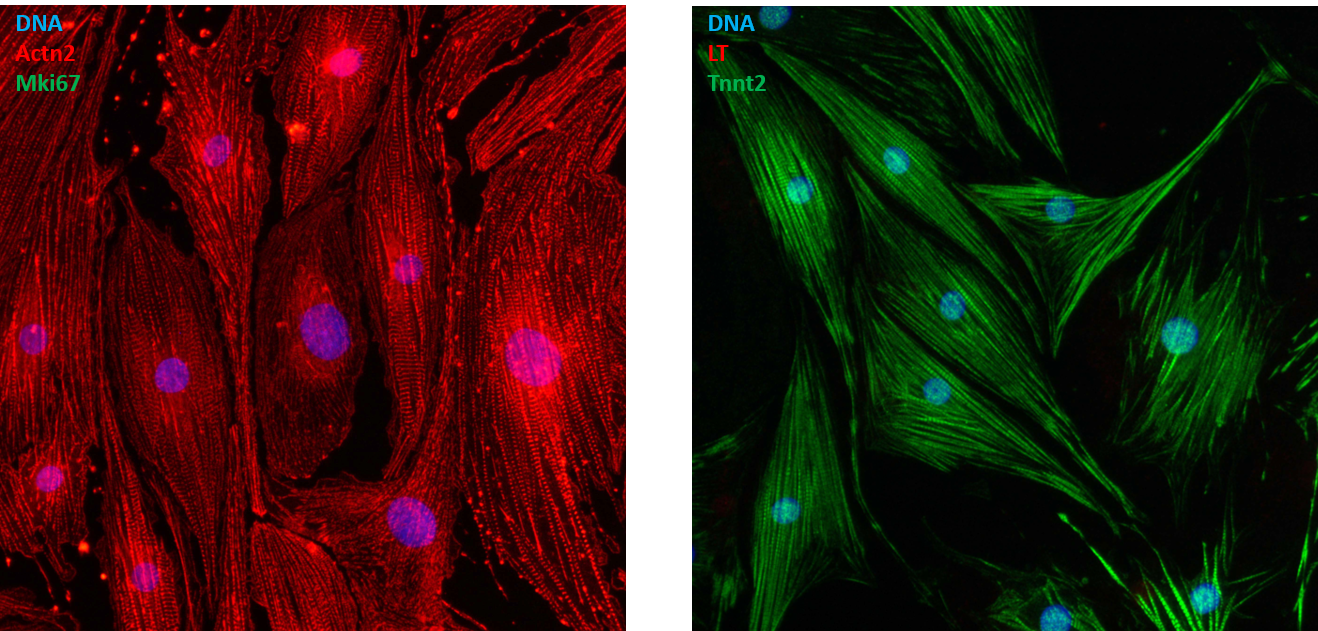
Figure: Cardiomyogenically differentiated iAM clone 315 cells double immunostained for sarcomeric α-actinin (Actn2) and the proliferation marker Ki-67 (Mki67; left panel) or for SV40 LT and cardiac troponin T (Tnnt2).
Encouraged by the highly promising results obtained with rat atrial myocytes, we decided to investigate whether it would also be possible to generate lines of conditionally immortalized human atrial myocytes with preserved cardiomyogenic differentiation ability. Because of the difficulty to obtain neonatal tissue for this purpose, we decided to use second trimester abortion material as source of atrial myocytes. Similar to our findings with neonatal rat atrial myocytes, the transduction of fetal human atrial myocytes with a lentiviral vector conferring doxycycline-dependent expression of LT gave rise to cells that could be massively amplified in the presence of doxycycline and differentiated into excitable and contractile cardiomyocytes in the absence of the drug [10]. By analogy with what we did for the conditionally immortalized neonatal rat atrial myocytes, the conditionally immortalized fetal human fetal atrial myocytes were called hiAMs standing for human immortalized Atrial Myocytes.
Although the iAMs and hiAMs represent very attractive models for fundamental and translational research into atrial biology and disease, the cells display subtle differences compared to their primary counterparts and in terms of phenotypic maturity are still far away from the brick-shaped atrial myocytes found in adult mammals. Moreover, for studies of ischemic heart disease, heart failure, ventricular tachycardia, sudden cardiac death and cardiotoxicity, one would preferentially like to use conditionally immortalized ventricular myocytes. This raises several interesting questions for future research:
- Does our conditional cell immortalization technology also allow the generation of differentiation-competent lines of adult mammalian atrial myocytes and, if so, will the differentiated end products possess a higher degree of phenotypic maturity than obtained thus far?
- Is it be possible to (further) increase the phenotypic maturity of iAMs and hiAMs by physical and/or chemical stimuli?
- Does our conditional cell immortalization technology also allow the generation of differentiation-competent lines of mammalian ventricular myocytes?
- Would the use of early gene products of polyomaviruses different from SV40 or mutant versions of SV40 LT, 17kT and small t (st) for the conditional immortalization of mammalian cardiomyocytes yield differentiation-competent cell lines with better (functional) properties or at a higher frequency?
Irrespective of the desire to push the conditional immortalization technology further forward and to generate differentiation-competent lines of additional types of cardiomyocytes, recent experience of us [11,12] and fellow scientists testifies of the utility of iAMs as cardiac research model. We expect this to be even more the case for the hiAMs due to their human origin and therefore higher clinical relevance.
Animation video
For an animation movie explaining in layman's terms how the hiAMs were generated and what one can do with these cells, see below.
References
- Nguyen PD et al. Enrichment of neonatal rat cardiomyocytes in primary culture facilitates long-term maintenance of contractility in vitro. Am J Physiol Cell Physiol 2012;303:C1220-8. URL https://doi: 10.1152/ajpcell.00449.2011.
- Bingen BO et al. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc Res 2014;104:194-205. URL https://doi: 10.1093/cvr/cvu179.
- Bingen BO et al. Atrium-specific Kir3.x determines inducibility, dynamics, and termination of fibrillation by regulating restitution-driven alternans. Circulation 2013;128:2732-44. URL https://doi: 10.1161/CIRCULATIONAHA.113.005019.
- Bingen BO et al. Constitutively active acetylcholine-dependent potassium current increases atrial defibrillation threshold by favoring post-shock re-initiation. Sci Rep 2015;5:15187. URL https://doi: 10.1038/srep15187.
- Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science 1988;239:1029-33. URL https://doi: 10.1126/science.
- Claycomb WC et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 1998;95:2979-84. URL https://doi: 10.1073/pnas.95.6.2979.
- Rybkin II et al. Conditional expression of SV40 T-antigen in mouse cardiomyocytes facilitates an inducible switch from proliferation to differentiation. J Biol Chem 2003;278:15927-34. URL https://doi: 10.1074/jbc.M213102200.
- Zhang Y et al. Controllable expansion of primary cardiomyocytes by reversible immortalization. Hum Gene Ther 2009;20:1687-96. URL https://doi: 10.1089/hum.2009.057.
- Liu et al. Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity. Cardiovasc Res 2018;114:1848-1859. URL https://doi: 10.1093/cvr/cvy134.
- Harlaar N et al. Conditional immortalization of human atrial myocytes for the generation of in vitro models of atrial fibrillation. Nat Biomed Eng 2021; URL https://doi: 10.1038/s41551-021-00827-5.
- van Ouwerkerk AF et al. Identification of atrial fibrillation associated genes and functional non-coding variants. Nat Commun 2019;10:4755. URL https://doi: 10.1038/s41467-019-12721-5.
- van Ouwerkerk AF et al. Identification of functional variant enhancers associated with atrial fibrillation. Circ Res 2020;127:229-243. URL https://doi: 10.1161/CIRCRESAHA.119.316006.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in