
Lithium-ion batteries enabled the portable technology revolution of the past two decades and are poised to power the conversion from internal combustion engines to electric vehicles. As the applications of energy storage diversify, so must the underlying battery materials. In our contribution to Nature in July 2018 (https://www.nature.com/articles/s41586-018-0347-0), we reported on two complex niobium tungsten oxide structural families that exhibit unusually rapid lithium diffusion, which enable charge and discharge through large particles in just minutes. Bulk particles are not generally capable of operating under high current density conditions due to limited solid-state diffusion; however, they have numerous practical advantages for battery operation, including ease and low cost of synthesis and minimal reactivity with the electrolyte.
The motivation for looking at these niobium tungsten oxides came directly from our previous fundamental study on the polymorphs of niobium pentoxide (J. Am. Chem. Soc. 2016, 138, 8888–8899). That research led us to explore the literature and crystal structure databases for phases with related structural motifs, which we discovered in ternary niobium-based oxides of e.g. tungsten and titanium. The complex atomic arrangements intrigued crystallographers including Wadsley, Roth, Anderson, Andersson, Hyde, Allpress, Lundberg, and Stephenson throughout the 1960s and early 1970s but no applications emerged at the time. As a result, these structure families mostly fell out of the primary research literature yet made their way into some popular crystal chemistry textbooks (e.g. Solid State Chemistry and its Applications – Anthony West, 1987 and 2014 eds.; Structural Inorganic Chemistry – Wells, 1984).
It was a four year journey from synthesizing a series of these complex oxides to the publication of our article in Nature. Beyond studies of the electrochemical performance, we were interested many mechanistic questions: How do the niobium tungsten oxides store charge beyond the conventional one electron redox process? How fast is the lithium ion conduction? How do the local and global structure respond to lithium insertion and extraction so rapidly and reversibly? What can these structures teach us about favourable ionic conduction more generally? First author Kent spent a lot of time at synchrotron X-ray sources in the US and UK with beamline scientists and co-authors Kamila Wiaderek and Giannantonio Cibin trying to answer these structural questions (Figure 1). High-rate electrochemistry measurements indicated that lithium moves fast through the niobium tungsten oxides but a breakthrough in quantitatively characterizing the lithium mobility came through the spatially-resolved NMR experiments. Co-author Lauren Marbella was able to perform challenging pulsed field gradient NMR experiments on these mixed ionic–electronic conductors, which revealed lithium self-diffusion faster than that measured in any lithium-ion battery electrode. Putting it all together (Figure 2), the niobium tungsten oxides exceptional energy storage capabilities come from the presence of (i) ideal interplanar spacing for lithium mobility, (ii) frustrated polyhedral networks that are not able to locally rotate/tilt in response to lithiation, and (iii) transition metals that can undergo multielectron redox with Nb(V)–Nb(III) and W(VI)–W(IV).
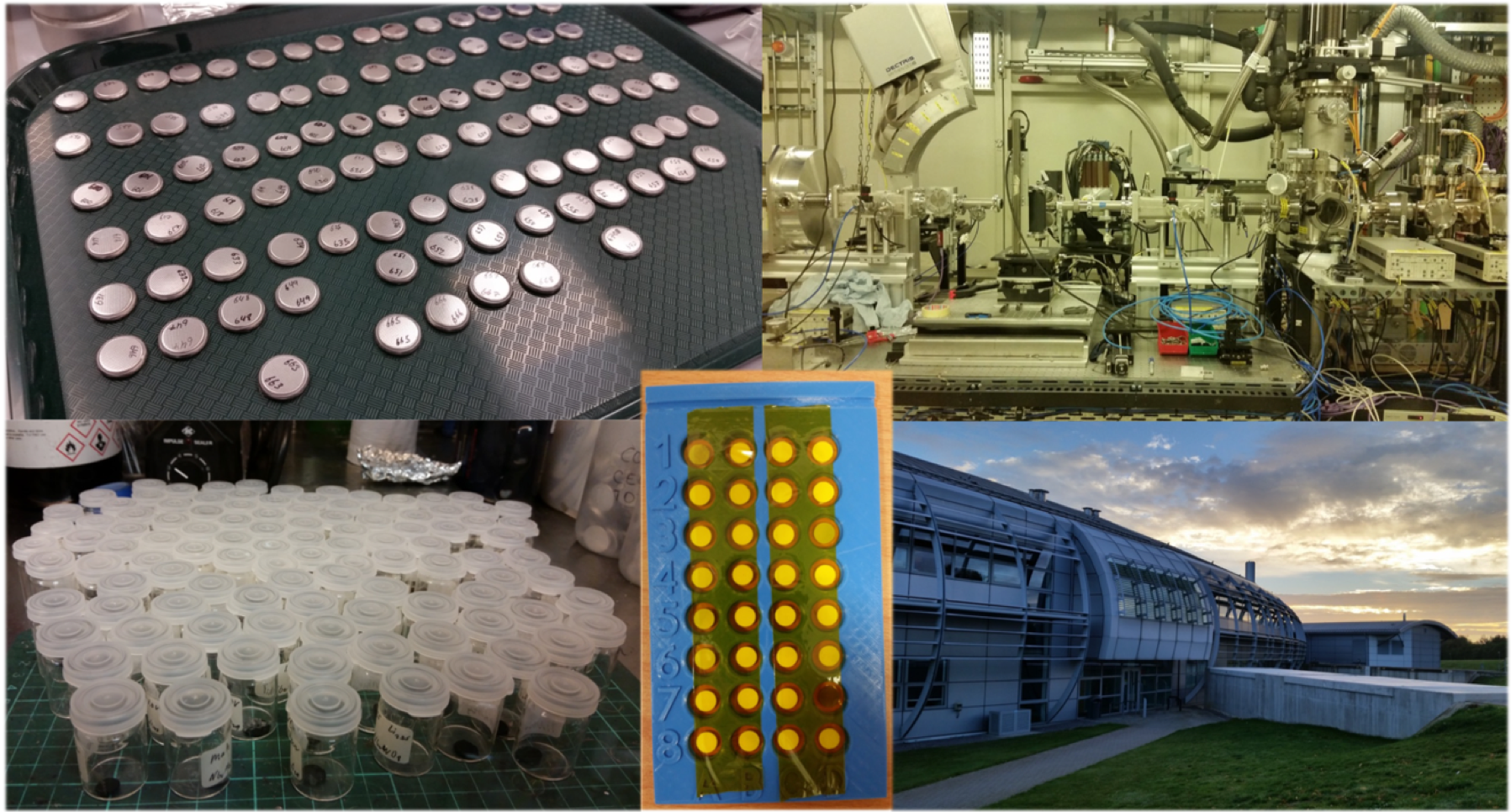
Figure 1 – Synchrotron X-ray Absorption Spectroscopy. Sample preparation from coin cell batteries (upper left) to extracted electrodes (lower left) to beamline samples (lower middle), Diamond beamline B18 instrumentation (upper right), and sunrise over Diamond Light Source (lower right).
Prior to publication, a patent was filed to protect commercialization of the materials and their application to energy storage. We have since established a Cambridge, UK-based start-up to help develop and licence the technology and to explore how this material compares with other anode materials that operate at similar voltages. Virtually all applications would benefit from faster charging, and the impact could be particularly significant for sectors such as electric cars and buses, household electronics, and warehouse vehicles.
Developing a new battery requires that the materials supply chain is worked out. We hope that this will be helped by our paper winning the Charles Hatchett Award from the Institute of Materials, Minerals & Mining (IOM3) “For the best paper on the science and technology of niobium-based materials.” (http://www.charles-hatchett.com/news/2019-charles-hatchett-award-winners-announced). The award is funded by CBMM – the Brazilian niobium mining company, helping to improve our contacts with the source of Nb. This award is now in its 41st year – an auspicious year for niobium, situated in the 41st spot in the periodic table!
The period following publication has been dynamic and exciting. First, it was well-received by the general and multidisciplinary technical news media including The Guardian, The Times, Newsweek, IEEE Spectrum, Chemistry World, Physics World, radio and TV interviews. The work appeared as an APS (Advanced Photon Source) Science Research and Engineering highlight (2018, Vol. 1) and was selected to highlight Beamline B18 in the Diamond Light Source 2018/19 Annual Review. We are now wrapping up an applied study of full cell battery performance combining our niobium tungsten oxide anodes with commercial high rate cathode materials using EPSRC funding designed to help translate research discoveries toward commercial realization. We are looking for new applications for these classes of materials and exploring how the behave under extreme – at least for batteries – cycling conditions. The research also spurred studies from other groups, some of which are emerging now on related structures, microstructural engineering, and specialized electrode formulation/applications. The future looks bright for these nearly-forgotten complex oxides.
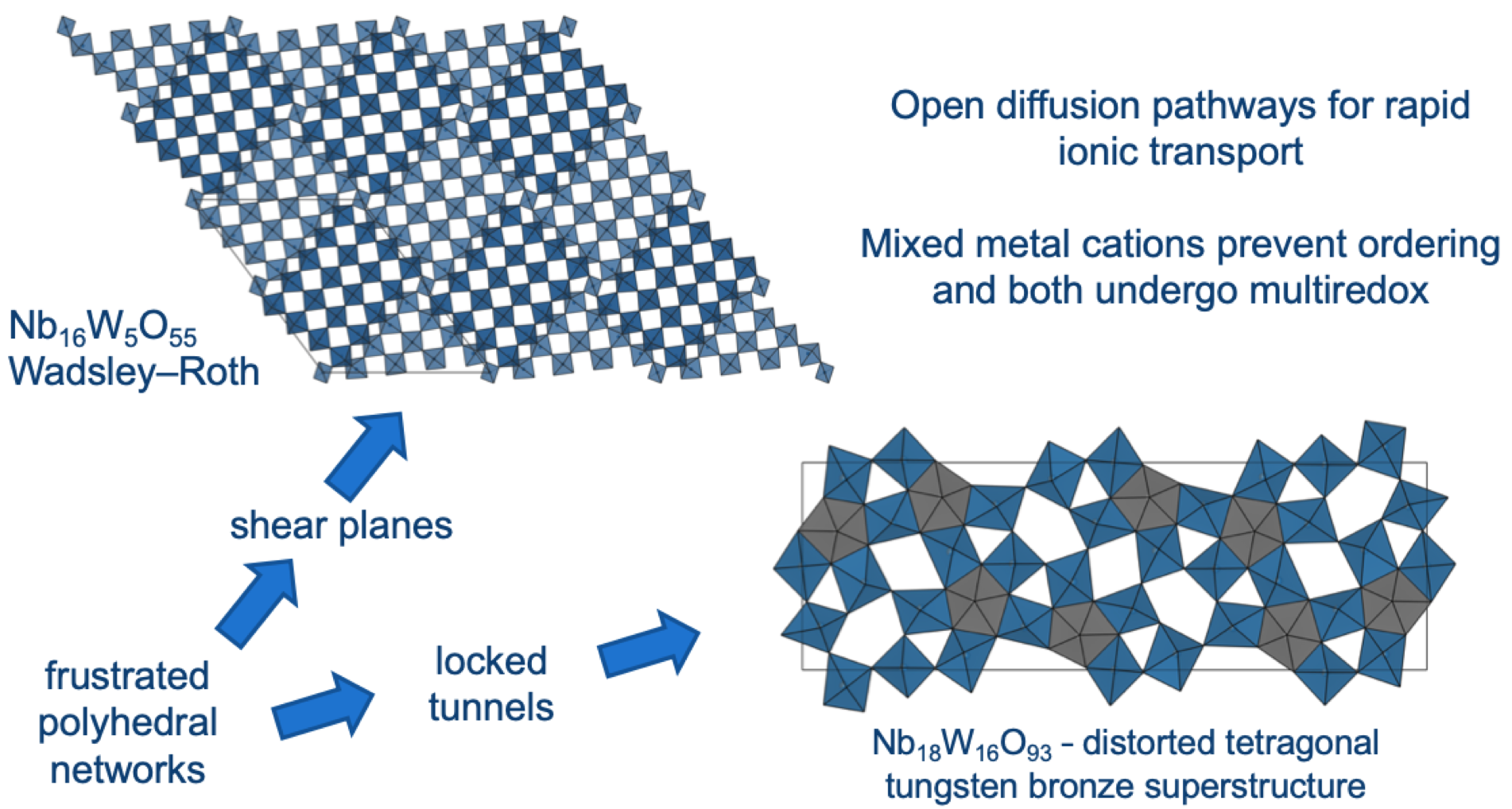
Figure 2 – Schematic of the key features of the niobium tungsten oxide compounds with Wadsley–Roth crystallographic shear (upper right) and bronze-like (lower left) structure types.
Kent J. Griffith and Clare P. Grey, June 2019
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in