Hidden Voids, Fast Track: How sulfides Vacancies Accelerate Critical Metal Mobility and Ore Formation
Published in Earth & Environment
-
The Motivation Behind This Research
Geologists know that for a hydrothermal deposit to form, trace critical metals must first be efficiently extracted from source rocks. These metals then travel through hydrothermal fluids before finally accumulating in a concentrated rock volume—one that’s accessible for effective recovery. They used to think that metal atoms move too slowly within minerals for solid-state diffusion (SSD) to play a major role in element transport. Instead, the widely accepted idea that fluid-induced coupled dissolution-reprecipitation (FI-CDR) efficiently releases and redistributes trace elements in mineral-fluid systems (Fig. 1).
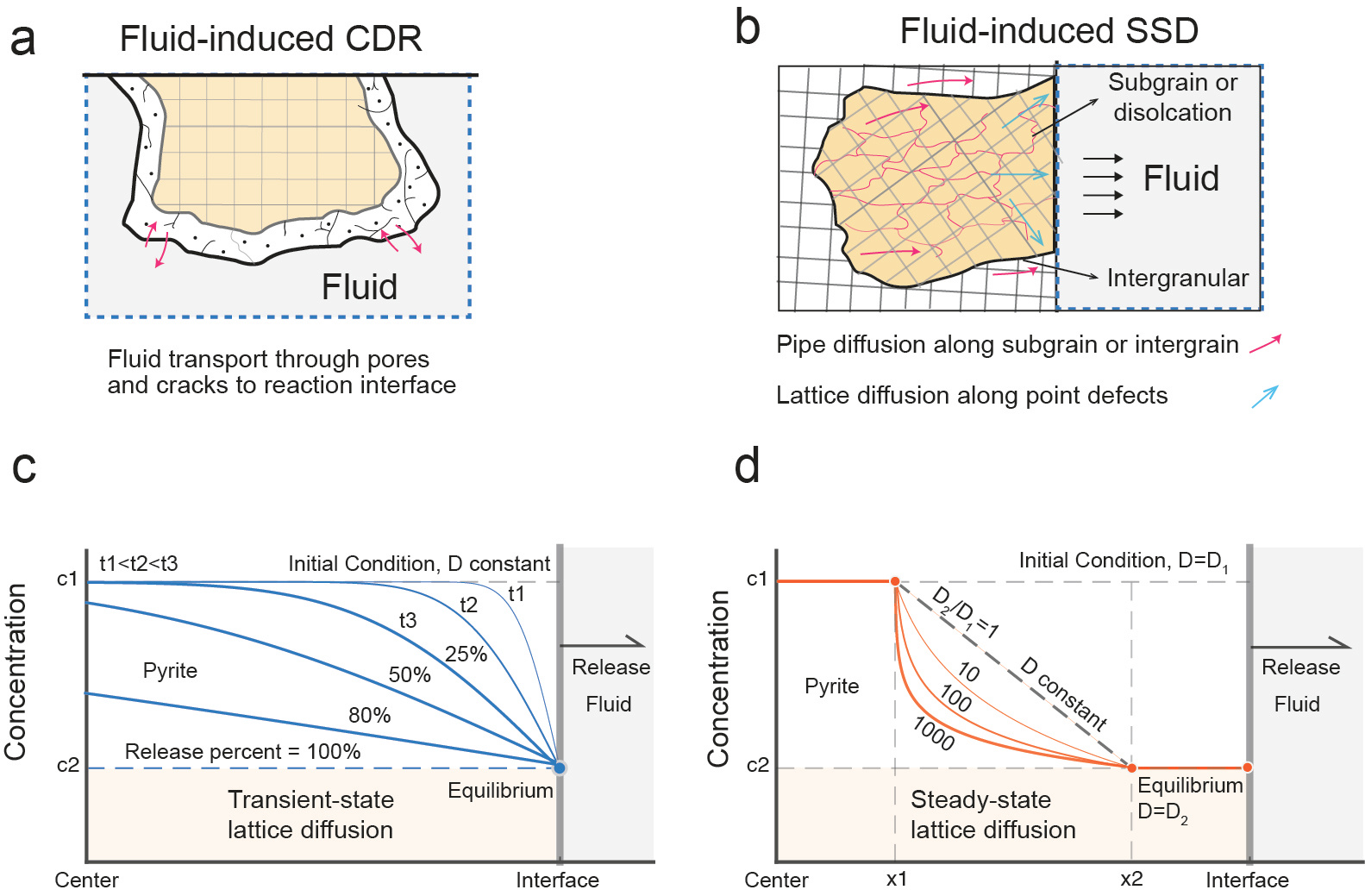
However, new experiments challenge this idea. They show that in sulfide minerals, fluids can trigger solid-state diffusion much faster than expected. Despite this exciting discovery, researchers still don’t fully understand how this rapid diffusion works, and they have yet to find clear evidence in natural samples.
As we report in Nature Communications, our study reveals that vacancies in natural pyrite act as fast pathways for element diffusion, allowing fluid-induced solid-state diffusion to occur nearly two orders of magnitude faster than traditional lattice diffusion. This process plays a crucial role in concentrating critical metals during ore formation, shedding new light on how valuable resources accumulate in Earth's crust. It potentially influences future exploration and extraction strategies.
-
What is fluid-induced solid-state diffusion?
In lattice diffusion, image a crowded room where everyone is packed tightly together. The particles are stuck in a rigid “grid” or “lattice” within the material, so their movement is slow and restricted—like trying to shuffle through a packed crowd.
Now, imagine fluid channel connected to this crowded room. As particles move into the channel, the room starts to clear out, allowing the particles to flow more freely and quickly. This is why fluid-induced solid-state diffusion is much more efficient—particles can move faster due to the added space.
This is different from pipe diffusion, where particles flow through the linear dislocations within the material—much like water flows through pipes. In pipe diffusion, the particles have clearer paths to follow, allowing them to move more freely and quickly.
-
Invisible Ghosts: Hidden Vacancies in Pyrite
Pyrite crystals can show dislocations or linear defects, which are visible under standard microscopes. However, vacancies in pyrite—tiny missing atoms in the crystal lattice, require sophisticated scientific tools to detect.
We observed Co-bearing pyrite at atomic resolution using an aberration-corrected scanning transmission electron microscope (Fig. 2). Our research found that large variations in the intensity of Fe columns can be attributed to vacancies (missing atoms) in the Fe columns, as shown by simulation calculations. This is especially common in Co-Ni doped natural pyrite, where vacancy-enhanced lattice diffusion occurs. Here, vacancies cluster together, creating fast pathways for cobalt transportation.
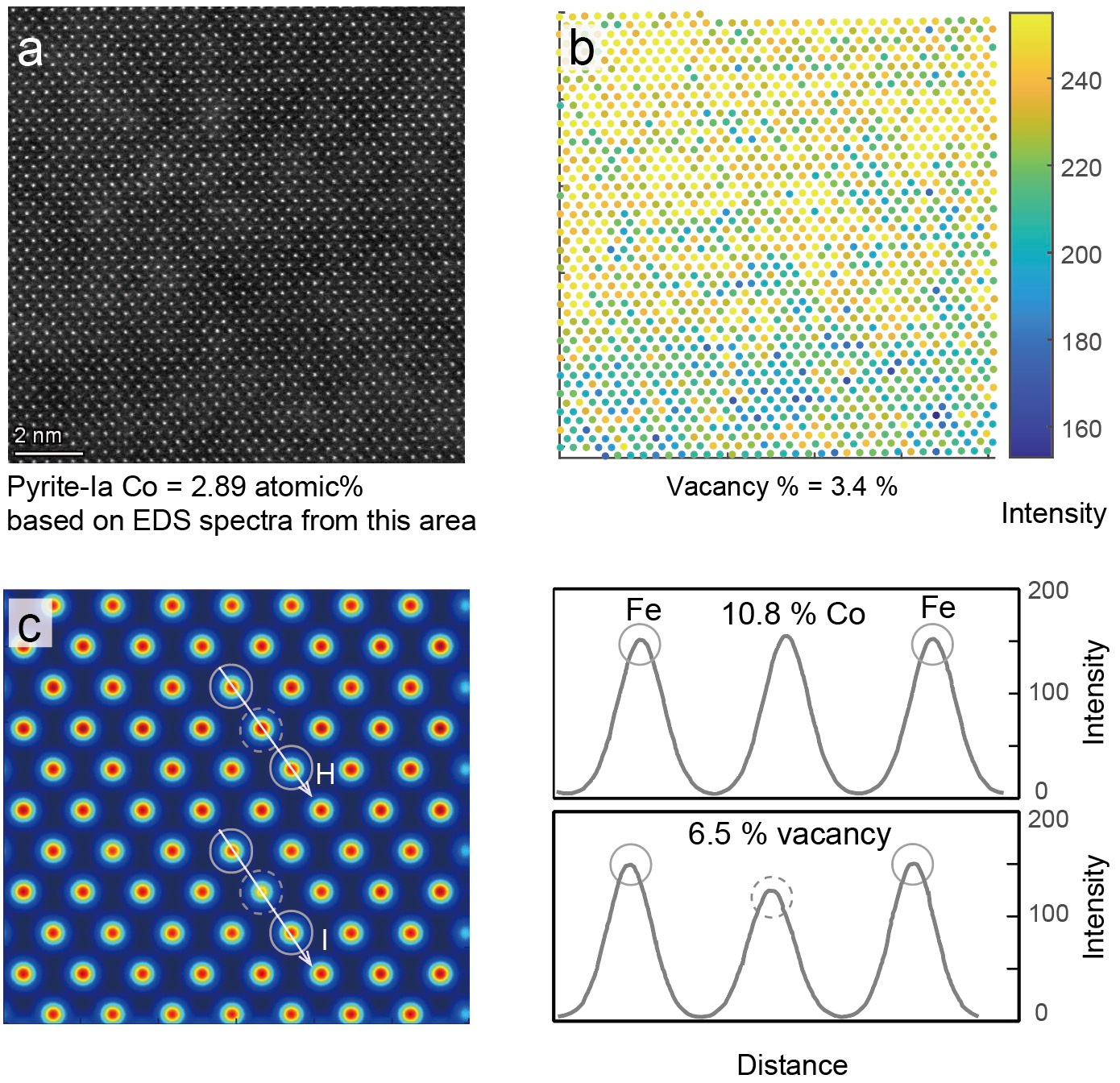
Fig. 2 Evidence for Fe vacancies in Co-bearing pyrite at atomic resolution.
a HAADF STEM images the Co-rich pyrite-Ia viewing along the (101) projection vector. b Intensity maps of Fe columns derived from the contrasts in HAADF image. c Simulated STEM image model of Fe columns and neighboring sites with 10.8 % Co atoms and 6.5 % vacancies, respectively.
-
Steady-State Diffusion: 1000 Times Faster Than Transient-State in Pyrite!
The transport of critical metals through vacancies channel can be mathematically described using steady-state diffusion, where the concentration profile does not vary over time and the flux J keeps constant in every position, according to Fick’s law. Steady-state diffusion is often overlooked in natural samples because its concentration profiles are typically sharp and narrow, making it harder to identify. The lattice diffusion is generally described by transient-state diffusion, where the concentration profile evolves over time, and the diffusivity remains constant (). Diffusion modeling show that the steady-state lattice diffusion process is faster than the transient-state diffusion process by 2 to 3 orders of magnitude, in terms of metal release flux rate (Fig. 3).
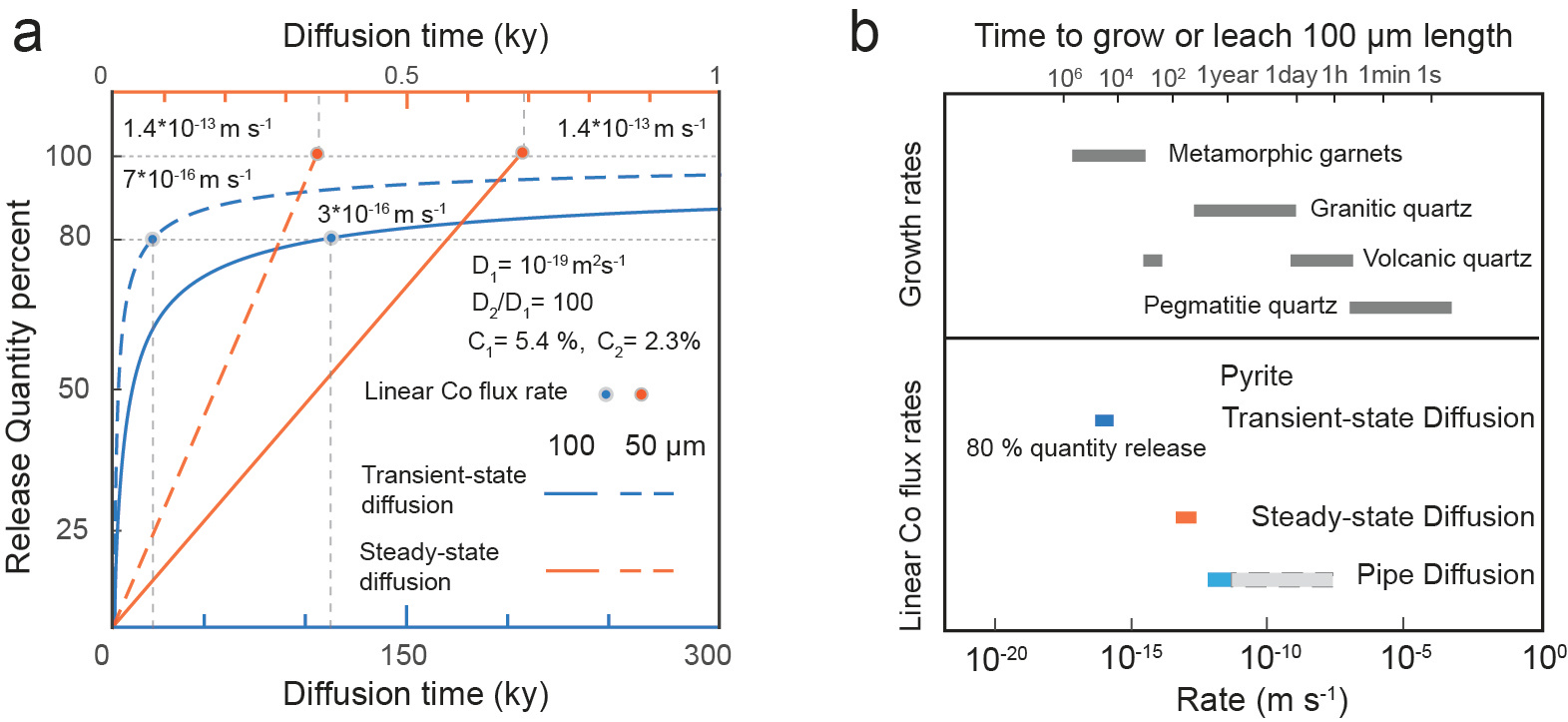
Fig. 3 Efficiencies of metals release in the pyrite-fluid interaction system.
-
Future research
Our discovery shows that the steady-state FI-SSD can have rates comparable to pipe diffusion or even the FI-CDR. In particular, the rapid FI-SSD accelerated by vacancies in the sulfide can well explain the abnormal zonation, exsolution and replacement often observed in natural sulfides. This breakthrough reshapes our understanding of how critical metals accumulate in ore deposits within Earth's crust. It potentially influences future exploration and extraction strategies. For example, cobalt resource in pyrite or sulfides can be extracted more efficiently through fluid leaching, without the need for oxidized in large reactors, which uses considerable amounts of energy.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in