HMGB2-induced calreticulin translocation required for immunogenic cell death and ferroptosis of cancer cells are controlled by the nuclear exporter XPO1
Published in Cancer, Protocols & Methods, and Cell & Molecular Biology

The paradox of oxaliplatin mediated immunologic cell death
We reasoned that an additional unidentified mechanism must be responsible for oxaliplatin induced ICD. Previous studies that examined oxaliplatin mediated secretion of HMGB1 did not determine whether HMGB2 was also secreted. This can be very challenging because the two proteins are 93 % homologous and so some antibodies cannot distinguish between them. Therefore, we used western blot analysis with highly selective antibodies together with mass spectrometry to show that oxaliplatin secretes both HMGB1 and HMGB2 from cancer cells (Figure 1).
HMGB2 secretion is controlled by the nuclear exporter protein exportin 1 (XPO1)
XPO1, also known as chromosomal region maintenance 1 (CRM1), mediates the nuclear export of various proteins and RNAs. We showed that the XPO1 inhibitors, leptomycin B and selinexor (KPT330), prevented the secretion of HMGB1 from lung cancer cells. This revealed that HMGB1 is another protein that undergoes XPO1-mediated nuclear export, which pointed us to the possibility that HMGB2 secretion is also mediated by XPO1. Using lung cancer and colon cancer cells we showed that selinexor also caused a significant reduction in oxaliplatin mediated HMGB2 secretion, together with a concomitant increase in nuclear HMGB2 (Figure 1).
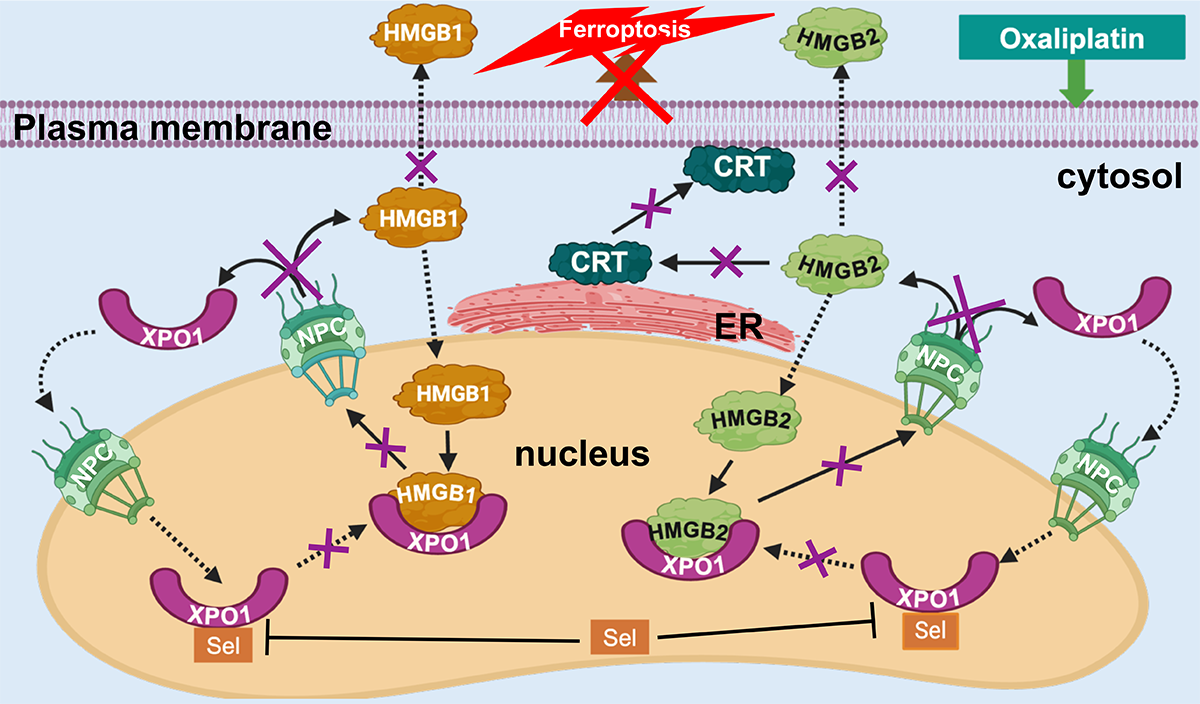
Figure 1: The consequences of XPO1 inhibition with selinexor (Sel) after incubating colon cancer cells with oxaliplatin (NPC = nuclear pore complex).
Oxaliplatin mediated HMGB2 secretion from the nucleus causes CRT translocation
HMGB2 (unlike HMGB1) is almost undetectable in the cytosol of both lung and colon cancer cells. The secretion of HMGB2 by oxaliplatin but not cisplatin, suggested that HMGB2 might be a mediator of oxaliplatin-induced CRT translocation. In support of this possibility, we found that inhibition of its secretion by selinexor prevented CRT translocation from the endoplasmic reticulum (ER) to the plasma membrane (Figure 1). Cell targeted CT-HMGB2 was efficiently taken up by both lung and colon-cancer cells. After 24-h no CT-HMGB2 was detected in the media and there was a dose-dependent increase of HMGB2 in the cytosol. This caused the translocation of CRT from the ER to the plasma membrane at concentrations that were almost three orders of magnitude lower than was required for oxaliplatin mediated CRT translocation.
A role for XPO1 in ferroptosis
A significant proportion of oxaliplatin-mediated HCT116 colon cancer cell death in vitro occurs through ferroptosis (Figure 1). Ferroptosis is a non-apoptotic type of iron-dependent programmed cell death, involving dysregulation of iron homeostasis and lipid peroxidation. It is characterized by decreased expression of glutathione peroxidase 4 (GPX4), increased production of reactive oxygen species (ROS), increased lipid hydroperoxide generation, increased FeII accumulation, and decreased nuclear factor erythroid 2-related factor 2 (Nrf2) expression. We observed that these five markers of ferroptosis were present after incubation of HCT116 cells with oxaliplatin. Furthermore, co-treatment of the cells with ferrostatin-1, which inhibits FeII-mediated generation of ROS, caused a 28 % decrease in oxaliplatin-mediated cell death. Inhibition of XPO1 with selinexor caused an even greater reduction in cell death (49 %) and reversed the five markers of ferroptosis that were observed with oxaliplatin alone.
HMGB1, HMGB2, and ferroptosis
Our study has provided compelling evidence that factors secreted from the nucleus are involved in the induction of ferroptosis. HMGB1 and HMGB2, which are secreted from the nucleus in response to oxaliplatin, are known to activate the receptor for advance glycation end-products (RAGE). Consequently, it is possible that secreted HMGB1 and HMGB2 can induce expression of nuclear factor kappa B (NF-κB). Inhibition of oxaliplatin-mediated secretion of HMGB1 and HMGB2 would prevent NF-kB-mediated down regulation of Nrf2 and inhibit both ROS- and lipid hydroperoxide-mediated ferroptosis. Therefore, inhibition of nuclear HMGB1 and HMGB2 secretion and/or preventing their activation of the NF-kB pathway, offers a potential therapeutic approach to preventing ferroptotic cell death in cardiovascular, pulmonary, and neurodegenerative diseases (4). Consequently, targeting the secretion of HMGB1 and HMGB2 or blocking their activity could lead to the discovery of novel therapeutic agents for treating these diseases.
HMGB2 and T cell exhaustion
It is noteworthy that immune checkpoint inhibitor (ICI) therapy synergizes with oxaliplatin cancer chemotherapy. Consequently, oxaliplatin and other chemotherapeutic drugs that induce CRT translocation, could improve the efficacy of ICI therapy for immune-resistant “cold” tumors. The improved potency of CT-HMGB2 for inducing CRT translocation when compared with oxaliplatin, suggests it could be a useful adjunct to ICI therapy. In addition, nuclear HMGB2 is involved in the differentiation and survival of functional memory cells as well as stem-like progenitor exhausted T cells (5). ICIs require the correction of T cell exhaustion to be effective, which is probably why they are only useful for treating a minority of cancer patients. Targeting HMGB2 might enable “cold” tumors to respond to ICI therapy by two distinct mechanisms: inducing CRT translocation and modulating T cell exhaustion. Therefore, novel proteins like CT-HMGB2 could complement current cancer therapies and expand the proportion of patients responsive to ICI-based therapies.
Bibliography
- Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28, 578-590 (2009). https://doi.org/10.1038/emboj.2009.1
- Tesniere, A. et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482-491 (2010). https://doi.org/10.1038/onc.2009.356
- Gillespie, K. P., Pirnie, R., Mesaros, C. & Blair, I. A. Cisplatin Dependent Secretion of Immunomodulatory High Mobility Group Box 1 (HMGB1) Protein from Lung Cancer Cells. Biomolecules 13, 1335 (2023). https://doi.org/10.3390/biom13091335
- Koutsodendris, N. et al. APOE4-promoted gliosis and degeneration in tauopathy are ameliorated by pharmacological inhibition of HMGB1 release. Cell Rep 42, 113252 (2023). https://doi.org/10.1016/j.celrep.2023.113252
- Neubert, E. N. et al. HMGB2 regulates the differentiation and stemness of exhausted CD8(+) T cells during chronic viral infection and cancer. Nat Commun 14, 5631 (2023). https://doi.org/10.1038/s41467-023-41352-0
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in