Homotrimeric architecture of viral G protein-coupled receptor UL78
Published in Cell & Molecular Biology
Herpesviruses encode G protein-coupled receptors (GPCRs) in their viral genomes and express these receptors in infected host cells to reprogram cell signaling networks of the host for survival, replication, and pathogenesis. As a ubiquitous herpesvirus with a seroprevalence of more than 50% in adults, human cytomegalovirus (HCMV) encodes four viral GPCRs (vGPCRs) including US27, US28, UL33 and UL78. The first three show about 30% homology to chemokine receptors (e.g., CXCR3, CX3CR1, CCR10 and CXCR1)1, whereas UL78 displays negligible homology to any endogenous receptors2. Besides the traditional vGPCR-mediated signalling paradigms including ligand-dependent signalling through a ligand−receptor−effector complex (as seen in US28) or ligand-independent, constitutive signaling via a receptor−effector complex (observed in US27), receptor hetero or homo oligomerizations have gained increasing experimental supports3,4. Notably, UL78 and UL33 can either form heterodimers with human CXCR4 and CCR5, interrupting normal signaling or presenting oligomers to modulate the functions of other vGPCRs and host GPCRs4,5.
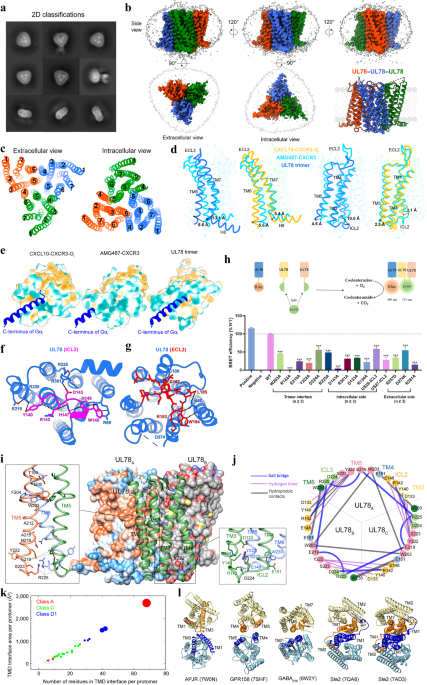
In this study, we report the cryo-electron microscopy (cryo-EM) structure of UL78 at a resolution of 3.12 Å and unveil a novel form of GPCR oligomerization unseen before − a homotrimeric architecture (Fig. 1). The overall structure of UL78 comprises a homotrimer along the 3-fold symmetry axis with each protomer closely interacting with the other two (Fig. 1a-c). The inward movements of intracellular loop 2 (ICL2) and the intracellular end of transmembrane helix 7 (TM7) fulfill the receptor intracellular crevice, thereby hindering the insertion of the Gαi protein C terminus (Fig. 1d). Looking at the extracellular side, the top of orthosteric ligand-binding pocket was significantly covered by the inward-folded extracellular loop 2 (ECL2), while the latter forms many polar contacts with surrounding TMs (Fig. 1e).
UL78 forms a stable homotrimeric architecture through an extensive interface covering TMs 3-6, ICL1 and ICL2 with a large interface area (7,956 Å2) (Fig. 1f). A central triangle in the trimer was formed by the tightly packed TM5 from three protomers, while the extracellular and intracellular halves of TM5 were further clasped by TM4 and TM3 from one neighboring protomer, respectively. Additionally, TM6 stacks in parallel with TM4 from the adjacent protomer. Such a unique homotrimeric architecture of UL78 employs a maximal number of residues (68 positions) and four TMs to construct the highest transmembrane domain (TMD) interface area per protomer (2,680 Å2) among all reported GPCR structures including active and inactive Ste2 (fungal class D1 GPCR), classes A and C GPCRs.
In summary, we have discovered a homotrimeric architecture of GPCR (UL78) significantly different from all reported GPCR homodimer or heterodimer structures and illuminated a new and most compact TMD packing. Considering that its trafficking between cell surface and cytoplasm and its ability to heterodimerize with US28, CCR5 and CXCR4, both structural and functional studies on the heteromeric UL78 with US28/CCR5/CXCR4 in the presence or absence of G proteins are worth pursuing. Clearly, further exploration of the physiological significance of our discovery could expand the knowledge about GPCR biology.

Fig. 1 Cryo-EM structure of homotrimeric UL78. a Representative cryo-EM 2D classification averages of UL78 homotrimers. b Cryo-EM density map of UL78 homotrimer. The sharpened cryo-EM density map at the 0.23 threshold shown as a light gray surface indicates a micelle diameter of 10 nm. c Extracellular and intracellular views of transmembrane helices (TMs) show the interfaces of UL78 trimer. d Magnified view of intracellular loop 2 (ICL2) within the intracellular pocket and its interactions with TMs and ICL1. e Magnified view of extracellular loop 2 (ECL2) within the extracellular pocket and its interactions with TMs. f Homotrimer interface of UL78, where two protomers are displayed in surface representation, and one in cartoon. Magnified views of the tightly packed TM5 from three protomers (left) and the intracellular bottom of the trimer interface (right) are shown.
References
1 Vischer, H. F., Leurs, R. & Smit, M. J. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol Sci 27, 56-63 (2006).
2 Rosenkilde, M. M., Tsutsumi, N., Knerr, J. M., Kildedal, D. F. & Garcia, K. C. Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses. Annu Rev Virol 9, 329-351 (2022).
3 Tschische, P., Tadagaki, K., Kamal, M., Jockers, R. & Waldhoer, M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol 82, 610-619 (2011).
4 Tadagaki, K. et al. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood 119, 4908-4918 (2012).
5 Wagner, S. et al. The 7-transmembrane protein homologue UL78 of the human cytomegalovirus forms oligomers and traffics between the plasma membrane and different intracellular compartments. Arch Virol 157, 935-949 (2012).
Follow the Topic
-
Cell Discovery

This journal aims to provide an open access platform for scientists to publish their outstanding original works and publishes results of high significance and broad interest in all areas of molecular and cell biology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in