How does the RTS,S malaria vaccine work?
Published in Microbiology

By Lyriye Kurtovic and James Beeson
Malaria is caused by parasites of the Plasmodium spp. genus, which are transmitted to humans by infectious mosquitoes. Since 2000, there has been a significant reduction in malaria burden, largely attributed to the up-scaling of anti-malarial drugs and vector control measures. However, their effectiveness is threatened by the emergence and spread of drug and insecticide resistance. Furthermore, malaria burden still remains high and accounts for an estimated 200 million clinical cases and half a million deaths each year [1]. Collectively, this highlights the need for novel anti-malarial interventions, and a priory of the WHO and funding partners is to develop a highly efficacious and long-lasting malaria vaccine [2]. Currently, only the RTS,S vaccine candidate has progressed to Phase 3 clinical trials. This landmark study was conducted in children and infants across 11 study sites in sub-Saharan Africa, and RTS,S vaccination was ~30-50% efficacious (depending on age group) against clinical malaria in the first year after vaccination [3]. While these findings were promising, vaccine efficacy was still considered suboptimal overall, and relatively short-lived.
Efforts to improve RTS,S will be important for developing a next-generation vaccine with greater efficacy and longevity in target populations. This has been challenging because we do not completely understand what immune responses confer protection against infection and disease, and therefore lack strong correlates of protection. RTS,S is known to induce potent antibody responses to the target antigen, circumsporozoite protein (CSP), but the functions of these antibodies are largely unknown [4].
Therefore, to address these questions we investigated the functional mechanisms of RTS,S-induced antibodies, using samples obtained from a Phase 2b RTS,S clinical trial conducted in children resident in Mozambique [5].
We demonstrate that RTS,S vaccination induced antibodies that could interact with human serum complement proteins. This ability to interact with, or ‘fix’, complement is required to then activate complement, and we have previously established that antibody-complement activation can kill the malaria parasite in vitro [6, 7]. Furthermore, complement-fixing antibodies have been associated with protection against clinical illness in a longitudinal study of children naturally exposed to malaria [7]. Therefore, the induction of complement-fixing antibodies by RTS,S suggests these antibodies may contribute to vaccine-induced immunity and protection.
Although children received the same vaccine regimen, the magnitude of complement-fixing antibodies varied markedly among participants. Further investigation suggests this was partly explained by differences in the type of antibody acquired (IgG subclass), and the region of CSP recognized by these antibodies (epitope specificity). Our findings indicate that antibodies to the C-terminal region of CSP were involved in complement-fixation, which was interesting because antibodies this region have been understudied, and instead only antibodies to the central region are currently considered important for RTS,S-induced immunity. Additionally, participant age and level of malaria exposure also influenced the induction of complement-fixing antibodies. Strikingly, older children with more malaria exposure had less complement-fixing antibodies, compared to younger children. This was a significant finding, which demonstrated that age and malaria exposure can greatly influence vaccine immunogenicity and possibly vaccine efficacy, and needs to be considered when administering malaria vaccines to different populations. Finally we examined the durability of complement-fixing antibodies, and found them to rapidly wane only months after vaccination. This was mostly due to the waning of IgG1 and IgG3 subclasses, suggesting that if greater levels of these antibodies were induced that were more durable, complement-fixing activity may have also been longer-lived.
Our findings encourage further investigation of complement-fixing antibodies induced by RTS,S vaccination, and whether this response is a correlate of protection. Greater understanding of these immune responses can guide the development of a next-generation vaccine that induces potent functional immune responses, and confers high levels of protective efficacy that is long lasting.
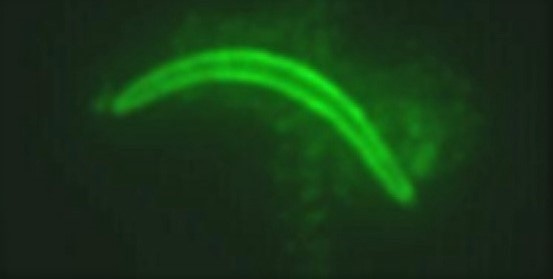
Image of a sporozoite, the target of the RTS,S malaria vaccine (Image copyright Bergmann-Leitner ES et al, PLOS ONE, 2010)
Poster image of vaccinated child - Robert Kapininga, a nurse assistant, gives baby Lusitana the first dose of the world’s first malaria vaccine. Photo: WHO/ M. Nieuwenhof
1. World Health Organisation, World malaria report 2017. 2017.
2. World Health Organization, Malaria vaccine technology roadmap. 2013.
3. RTSS Clinical Trial Partnerships, Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet, 2015. 386(9988): p. 31-45.
4. White, M.T., et al., Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. , 2015. 15(12): p. 1450-1458.
5. Alonso, P.L., et al., Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet, 2004. 364(9443): p. 1411-1420.
6. Behet, M., et al., The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect Immun, 2018: p. 00920-00917 7. Kurtovic, L., et al., Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med., 2018. 16(1): p. 61.
7. Kurtovic, L., et al., Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med., 2018. 16(1): p. 61.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in