
You have painstakingly grown (or, if you’re lucky, simply bought) a beautiful, large and shiny crystal of some interesting material. Probably the last thing you would think of doing is to clamp it in a vise and proceed to crush it beyond recognition. Yet it so turns out that this can be a very meaningful thing to do, and, by some measures, could even improve the material. If you’re starting to think that this is some sly conspiracy designed to get people to destroy their nice crystals, read on.
When people think of ceramics, they usually think of them as brittle – they break easily if you stress them. As countless encounters between coffee mugs and tiled floors have demonstrated, this is indeed mostly true. So, we were as surprised as anyone would be when, during tests of a new device that applies stress on small crystalline samples, we managed to (more or less by accident) plastically deform a ceramic material without shattering it. Instead of the expected brittle behaviour, the crystal acted a (little) bit like play-doh. And this was not just any material: it was one of the famous high-temperature superconducting cuprates, that lose all electrical resistance at temperatures upwards of 100 K. We of course became very interested in finding out what happens to the superconductivity in the deformed sample.
Now, in principle, a crystal is a perfectly periodic arrangement of atoms; although the real ones are never quite perfect, they can get pretty close. Yet to plastically deform a crystal, you necessarily have to mess it up – most commonly by introducing imperfections, or defects, known as dislocations. These are essentially entire planes of missing atoms in the structure, and during plastic deformation, they can form and move around in the crystal. If the dislocations are hard to form and move, the crystal cracks – the kind of brittle failure observed with mugs on floors. On the other hand, if you do manage to deform the sample plastically, it tends to be more disordered than the original, and disorder is typically thought to be bad for superconductivity. As you probably suspect by now, in our deformed cuprate crystal we found just the opposite – a slight enhancement of superconductivity (or, to be more precise, an enhancement of superconducting fluctuations). This was an eye-opening moment; yet that crystal was only deformed a little bit, and it turned out to be impossible to deform the material any further without turning it into dust. So, the obvious question was: is there a similar material that can be deformed a lot, and studied as a model system?
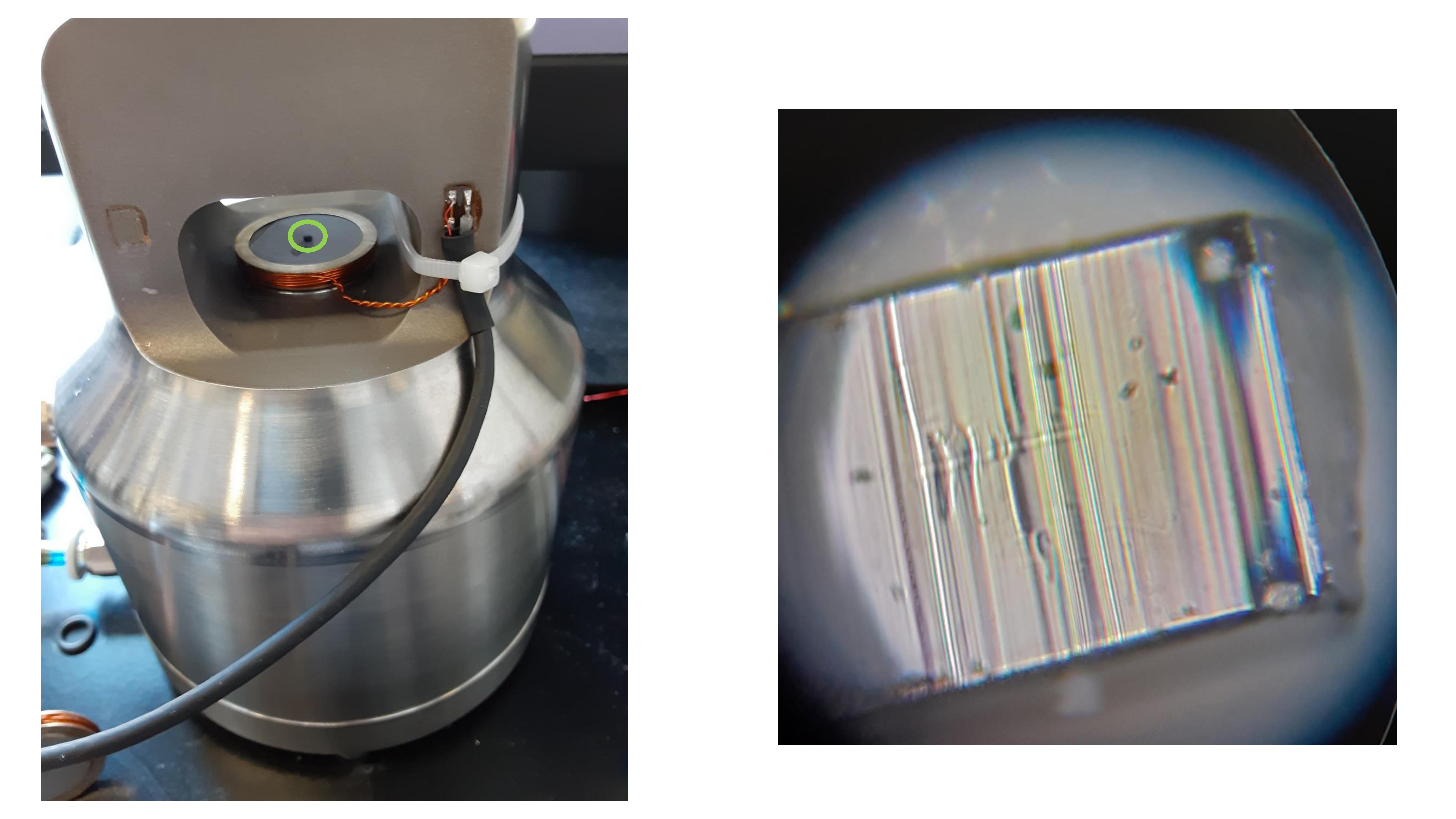
It turns out that there is, and it is also very famous (in the right circles) – strontium titanate (SrTiO3), known as STO. At room temperature, STO can be deformed by the astonishing amount of ten percent under compressive stress, while still looking more or less like a shiny crystal (figure 1). Even better, STO is widely used as a substrate for thin-film growth, so you can just buy crystals of it online; thus, instead of destroying priceless home-made cuprate samples, we quickly switched to destroying a lot of quite reasonably priced STO.
To back up a little: STO is a very important and interesting material indeed. At some point, it was the most popular fake diamond, and apparently far superior to the real thing in terms of light displays due to its strong dispersion. Perhaps more to the point, it is also the first oxide superconductor to be discovered – nearly six decades ago – and a major motivation in the research that led to the discovery of high-temperature superconductivity in the cuprates. Yet the superconductivity in STO is still not understood. Although the superconducting transition temperatures (Tc) are, one might say, a bit low in absolute terms (up to 0.4 K or so, very close to absolute zero), if you consider the ratio between the relevant electronic scale – the Fermi energy – and Tc, STO is the highest of high-Tc superconductors. It is therefore as good a model system as any to study the effects of plastic deformation on superconductivity.
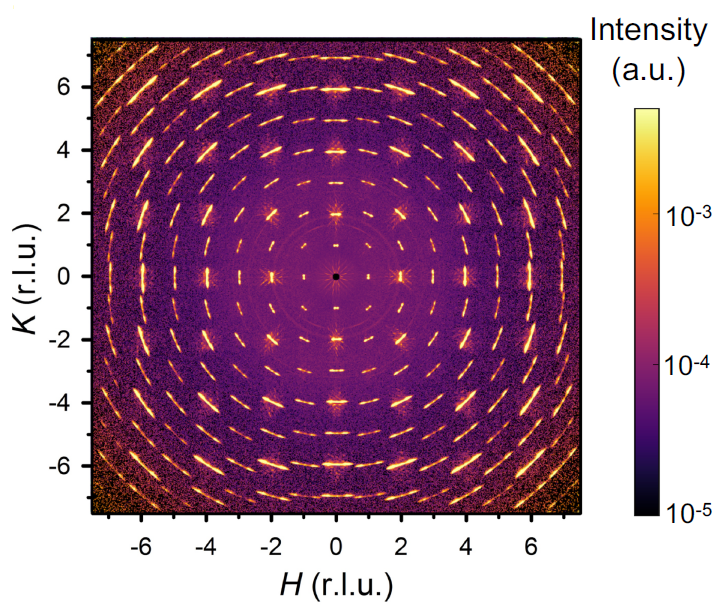
As described in our new paper in Nature Materials, the effects are substantial. First of all, plastic deformation causes only a modest increase in the number of dislocations in STO. Instead, the main structural effect is that the dislocations self-organize into large periodic structures known as dislocation walls. We managed to observe these using neutron scattering and X-ray, yielding some rather pretty pictures (figure 2). Around the walls, the electrical properties of the material change, and we believe that this leads to the observed superconductivity enhancement. In addition to the clear, roughly factor-of-two increase of Tc in deformed samples, there are also tantalizing signs of superconducting correlations at temperatures two orders of magnitude higher than that!
Now, it is tempting to imagine what extensive plastic deformation might do in other materials. How far could one push Tc in cuprates if there is a way to deform them substantially? Could plastic deformation be used to tune other properties, such as magnetism? Is it feasible to improve materials used for the generation of electricity – thermoelectrics – by deforming them? Possibilities abound, and people might start destroying their nice crystals after all…
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in