Hydrogen-bonding and π-π interaction promoted solution-processable covalent organic frameworks

Covalent organic frameworks (COFs), as a crystalline porous polymer, has demonstrated considerable potential in various fields, including energy storage, desalination, gas separation, catalysis, and organic optoelectronics. In these applications, solution processability is generally required to generate useful forms such as membrane, and it offers substantial cost advantages in processing while maintaining excellent material performance. However, COFs are mostly obtained as micro-sized or larger solid powders, which are not dispersed in common organic solvents and water. Consequently, processing them into the aforementioned forms using solution processes becomes challenging. This means that although COFs have excellent properties, they can only partially achieve their capabilities or even fail to realize their potential in practical implementation.
Given the advantages of solution processing, scientists have dedicated their efforts to developing solution-processable COFs. The challenge associated with COF’s dispersibility arises from the weak interaction between COFs and solvent molecules. One approach involves functionalizing COFs with long-chain structures such as long ether or alkyl chains. By modifying these functional groups, the interactions between COF and solvent molecules are enhanced, enabling the fabrication of membranes through solution processing. However, this bottom-up method is highly dependent on the COF structure, such constraint hinders the broad applicability of this method.
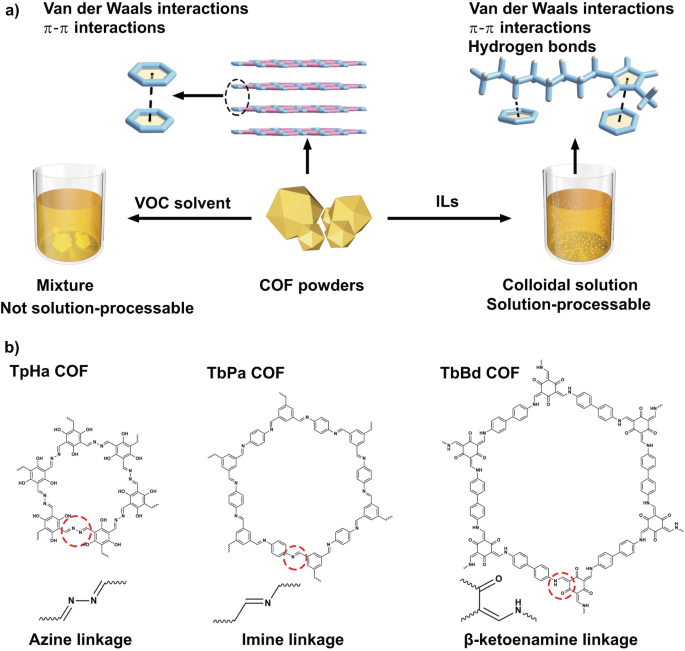
To develop a more universally applicable method for preparing solution-processable COFs, we consider the inherent structural characteristics of COFs. COFs are a class of typical two-dimensional materials with interlayer van der Waals and π-π stacking interactions, which are difficult to be broken by conventional solvents. The key factor in preparing solution-processable COF materials is to disrupt the interlayer interactions while preserving the dynamic linkage such as imine, azine, and β-ketoenamine. But identifying or designing solvents that meet the aforementioned criteria is not easy because we need to take into account various characteristics of solvent molecules, such as thermal stability, boiling point, charge distribution, and chemical compatibility with COFs.
Ionic liquids (ILs) are composed entirely of cations and anions. Compared to traditional organic solvents, they have a wider liquid range, better thermal stability, and, most importantly, contain abundant hydrogen bond donors and acceptors, as well as conjugated structures. These unique structures offer the potential for forming stable composite structures between ILs and other compounds. In our previous work, we demonstrated that ILs can disrupt relatively strong van der Waals interactions and C‒H···π interactions in the crystals of conjugated molecule pentacene, and showed good chemical compatibility with this conjugated structure, enabling the solution processing of insoluble aromatic compounds.
Therefore, we selected ILs as solvents to treat these difficult-to-disperse COF powders. To verify their universality, three types of COFs with imine, azine, and β-ketoenamine linkages were chosen. The synthesized pristine COFs were placed in IL 1-methyl-3-octylimidazolium bromide ([C8mim]Br) and heated for 24 hours. Subsequently, large particle COFs were separated by centrifugation, resulting in COF-IL colloid with a concentration of up to 0.95 mg·mL−1. Dynamic light scattering and scanning electron microscope (SEM) results showes that the particle size of COFs could be reduced to approximately 80 nm after heat treatment. Experimental evidence from powder X-ray diffraction (PXRD), Fourier transform infrared (FTIR) spectroscopy, and solid-state nuclear magnetic resonance (NMR) spectroscopy confirm that the crystal and chemical structure of COFs remain unchanged, and no decomposition products from COFs were detected in the ILs after heat treatment. These treated COFs can be dispersed well in common organic solvents ethanol, dichloromethane, acetone, and water, enabling solution processing.
To gain a deeper understanding of the interactions between COFs and ILs, which is crucial for preparing solution-processable COFs, we employed molecular dynamics (MD) simulations and quantum chemical calculations to investigate the interactions between ILs and COFs. The results of MD simulations revealed that anions have minimal interaction with the conjugated structure of COF but they can influence the interaction between cations and the COF based on their charge distributions and volumes. IL cations tended to distribute above and below the conjugated plane of COFs. Further Quantum Theory of Atoms in Molecules (QTAIM) analysis indicated that the IL cations could form π-π interactions and C‒H···π interactions with the conjugated structures in COFs. Therefore, we speculate that these non-covalent interactions are key factors in promoting formation of solution-processable COFs.
This study presents a top-down approach utilizing ILs as solvents for the preparation of solution-processable COFs. This method reduces the dependency on the COF structures. By exploiting the non-covalent interactions formed between the IL cations and COFs to assist in disrupting the inherent interlayer interactions of COFs, solution processing for COFs was achieved. Further research will prioritize the exploration of various applications to fully unleash the performance of these solution-processable COFs.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in