IMMUNOTHERAPY AS A POTENTIAL THERAPEUTIC OPTION IN THE MANAGEMENT OF EPITHELIAL OVARIAN CANCER
Published in Cancer

Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) is an accepted treatment for patients with advanced stage epithelial ovarian cancer (EOC) 1,2. Nevertheless, suitable post-surgery treatments in these patients are an unmet need. Despite initial chemosensitivity, most patients relapse and ultimately platinum resistance occurs, which results in a poor 5-year survival of 45% 3. In the hope of reaching the success seen in other cancer types, attention has turned to immunotherapy as a new strategy to improve survival in EOC patients.
In EOC, immunotherapy success in unselected patients has been modest with overall response rates of only 15%. As in many other tumor types, the challenge remains to determine which patients are likely to benefit and what treatment combinations are most suitable, taking into account current EOC management 4. Collectively, this highlights the urgent need for novel immunotherapy strategies and immune biomarkers to guide patient selection.
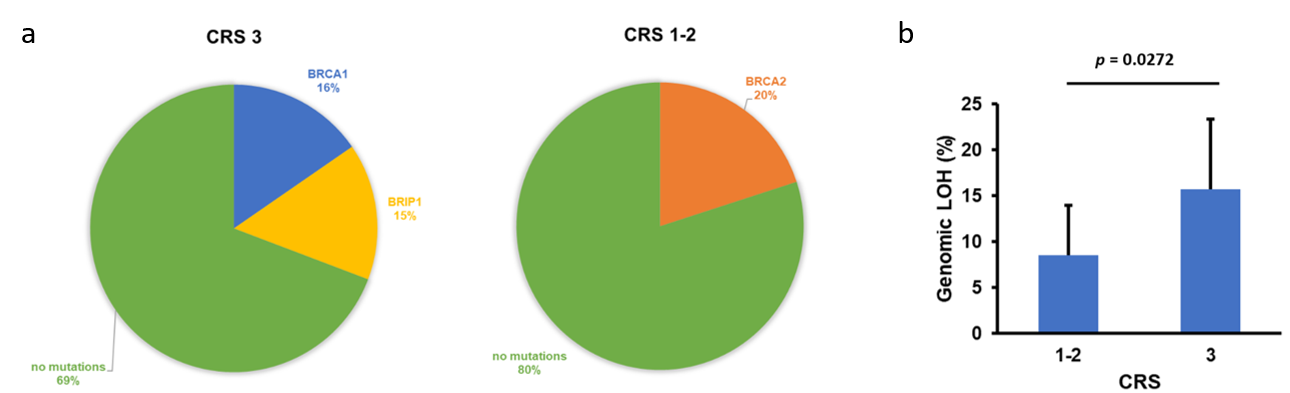
Homologous recombination deficiency (HRd) is known to play an essential role in cancer progression and better responses to chemotherapy have been described for HR-deficient tumors 5. In our cohort, which consisted of 60 EOC patients with sufficient viable tumor content in biopsy (before undergoing NACT) and/or IDS samples (after receiving NACT), we found a non-significant increase of HR gene alterations in NACT responder patients (Figure 1a). Of note, the value of HRd as a biomarker can be measured by quantifying the genomic LOH 6. The extent of genome-wide LOH may identify BRCA wild-type patients with HR-deficient tumors who have other alterations not recognizable by the assessment of short mutations in HR pathway genes. As these tumors usually show sensitivity to poly-ADP polymerase inhibitor (PARPi) therapy, the use of LOH as a biomarker might be of particular interest in HR-deficient tumors. In our study, we found a significantly higher genomic LOH in responders to NACT than in non-responders, which is in agreement with previous reports (Figure 1b) 7.
Besides, our observations that immune states undergo changes during NACT treatment underscore the dynamic nature of tumor-host immune interactions. Our data indicate that immune dynamics change during chemotherapy, since the immune gene expression profile after NACT revealed activation of several immune regulation-related pathways in patients with no/minimal or partial response (Figure 2).
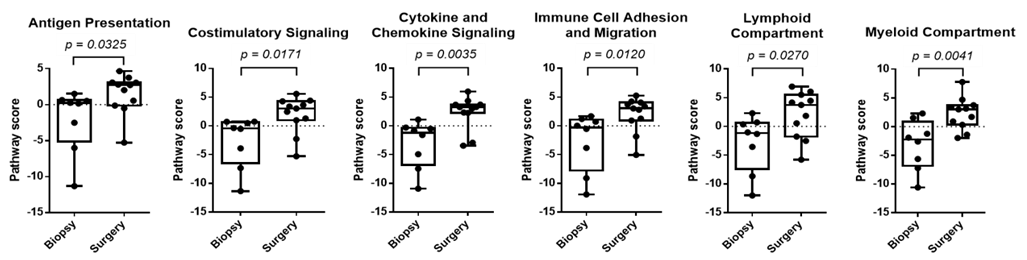
Figure 2. Increase of inflammatory signature in EOC surgery samples from patients with no/partial response to NACT. Several pathways associated to immune regulation are stimulated by NACT.
At the same time, NACT caused a significant increase of the tumor infiltrating lymphocyte population, primarily due to an enhancement of the CD8+ T cell population, in this patient population (Figure 3). Remarkably, these changes occurred irrespective of potential HR defects, such as t hose associated to BRCA1/2 mutations.
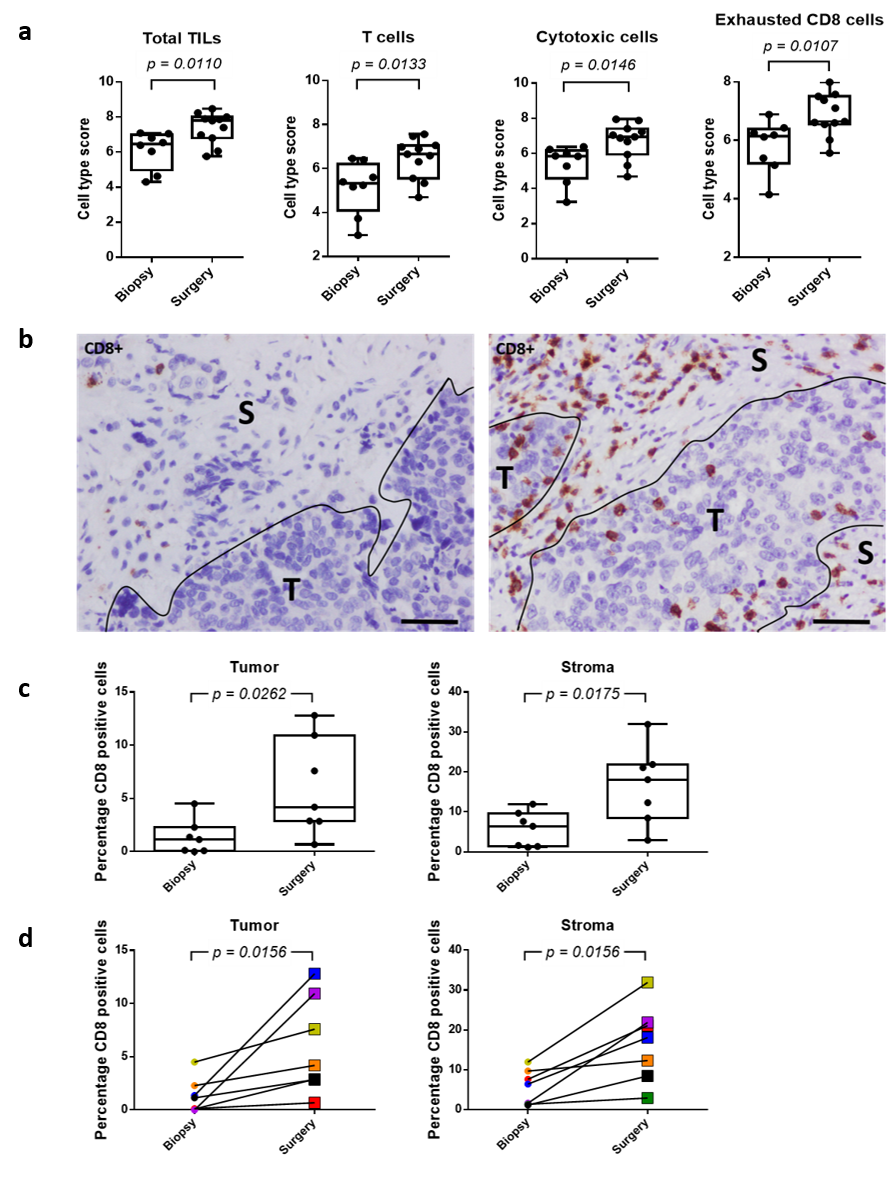
Figure 3. NACT enhances immune cell abundance and CD8+ cell infiltration in both tumor and stromal areas of the EOC sample. a) Different immune cell subsets show increased abundance in surgery samples after NACT. b) Immunohistochemistry showing the presence of CD8+ cells in EOC sample before (left) and after (right) NACT. S stroma, T tumor. Scale bars, 100 μm. c) Plot of percentage of CD8+ cells in tumor and stromal areas before (biopsy) and after (surgery) NACT. d) Line graph representing the percentage of CD8+ cells in tumor and stromal areas in paired biopsy-surgery samples. Each line represents an EOC patient, with colors defining the same patient in both analyses.
In order to make accurate predictions with regard to therapeutic response and the possibility to design potential new therapeutic strategies with immune checkpoint inhibitors (ICIs), larger longitudinal studies will be needed to monitor longitudinal changes. Specifically, our observations with regard to specific (immune) gene signatures and increased presence of effector immune cells, together with the increased expression of PD-1, indicate the potential use of ICIs after NACT in EOC patients with no/minimal or partial response to this therapy (Figure 2, 3 & 4). Of note, the analysis of immune gene signatures and immune cell abundance alone might not be sufficient to discriminate patients as candidates for ICI treatment.
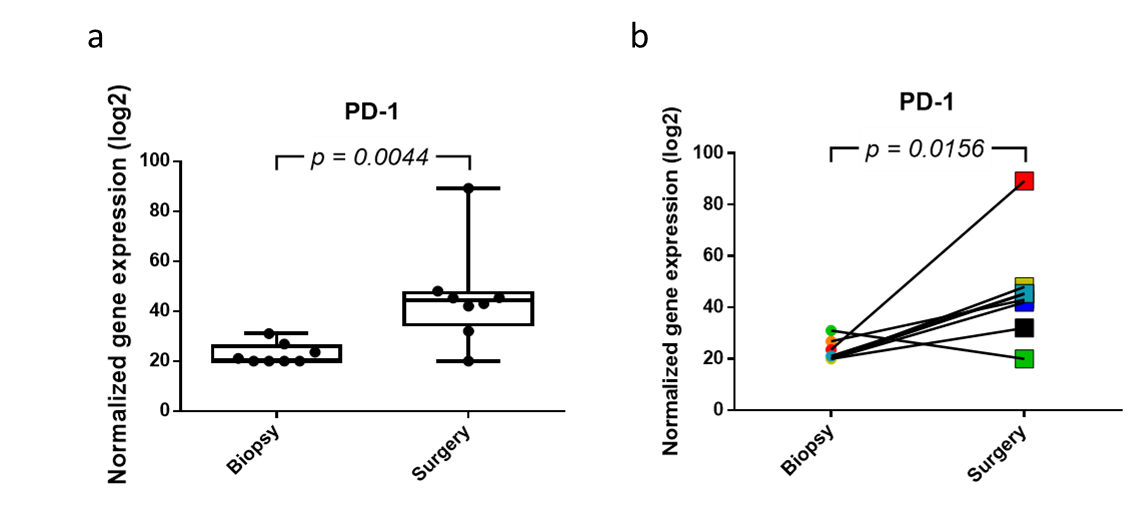
Figure 4. Normalized gene expression of PDCD1 (PD-1) before and after NACT. NACT causes a significant increase in PD-1 expression. a) Plot of PD-1 expression in which the value for each EOC sample is shown. b) Line graph representing the increase in PD-1 expression in paired biopsy-surgery samples. Each line represents an EOC patient.
Based on the results of this study, patients who show no/minimal or partial response to NACT might be beneficial candidates for the treatment with ICIs as NACT has been found to provoke a beneficial immune profile for subsequent treatment with immunotherapy in this patient population. However, the influence of other factors on the ICI response should be taken into account, like changes in tumor mutational burden (TMB) and mutation-derived antigens. With regard to TMB, we did not find any significant alterations between biopsy samples from responder and non-responder patients, which might be either due to the limited number of samples or a true absence of difference in TMB after NACT. Nevertheless, we demonstrated a clear change in TME in no/minimal and partial responder patients to NACT, showing a significant increase in immune cell infiltration and expression of PD-1. Even though this TME might indicate the potential efficacy of immunotherapy in this patient population, specific targets for ICIs remain to be identified.
Our study provides an important insight into evolution and mutational processes occurring in EOC and how these are being affected by NACT. Respectively, our findings strengthen the use of LOH as biomarker of HRd, which might be of particular interest with regard to PARPi therapy. Moreover, changes of immune states during NACT reveal the dynamic nature of tumor-host immune interactions and suggest the potential use of ICIs or their combination with PARPi in high stage and grade EOC patients undergoing NACT. Conclusively, our data can pave the way to personalized therapy and new therapeutic strategies in EOC management.
Interested to know more about our study, check out our full article: https://rdcu.be/cFOPN
- Colombo, N. et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 30, 672–705 (2019).
- Vergote, I. et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 363, 943–953 (2010).
- Coukos, G., Tanyi, J. & Kandalaft, L. E. Opportunities in immunotherapy of ovarian cancer. Ann. Oncol. 27, i11–i15 (2016).
- Hartnett, E. G. et al. Immunotherapy advances for epithelial ovarian cancer. Cancers 12, 1–27 (2020).
- Tumiati, M. et al. A functional homologous recombination assay predicts primary chemotherapy response and long-term survival in ovarian cancer patients. Clin. Cancer Res. 24, 4482–4493 (2018).
- Abkevich, V. et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 107, 1776–1782 (2012).
- Takaya, H. et al. Intratumor heterogeneity and homologous recombination deficiency of high-grade serous ovarian cancer are associated with prognosis and molecular subtype and change in treatment course. Gynecol. Oncol. 156, 415–422 (2020).
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
Tumor-type-agnostic biomarkers and treatments in oncology
Publishing Model: Open Access
Deadline: Mar 05, 2026
Emerging adjuvant and neo-adjuvant treatment approaches in solid tumors
Publishing Model: Open Access
Deadline: Mar 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in